Recombinant alkaline phosphatase for use in treating sepsis-associated acute kidney injury
A technology for sepsis and kidney injury, applied in the field of use of recombinant alkaline phosphatase for the treatment of sepsis-related acute kidney injury, and can solve the injury, loss of renal function, high cost, morbidity and mortality, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0273] Human recombinant alkaline phosphatase for sepsis-associated acute kidney injury
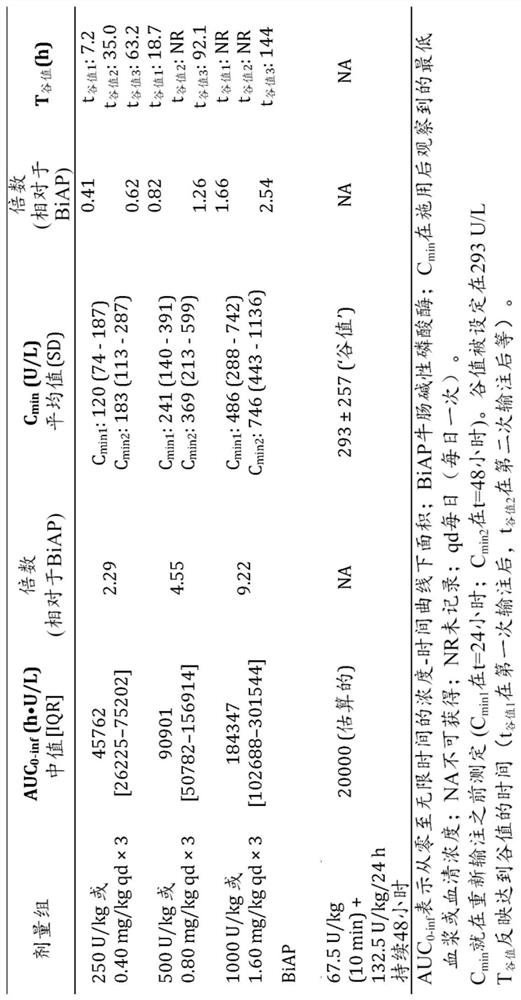
[0274] Sepsis-associated acute kidney injury (AKI) adversely affects long-term renal outcome and survival. In preclinical studies, administration of the detoxifying enzyme alkaline phosphatase improved renal function and survival. We studied human recombinant alkaline phosphatase (RecAP) in patients with sepsis-associated AKI ( figure 2 ) efficacy and safety.
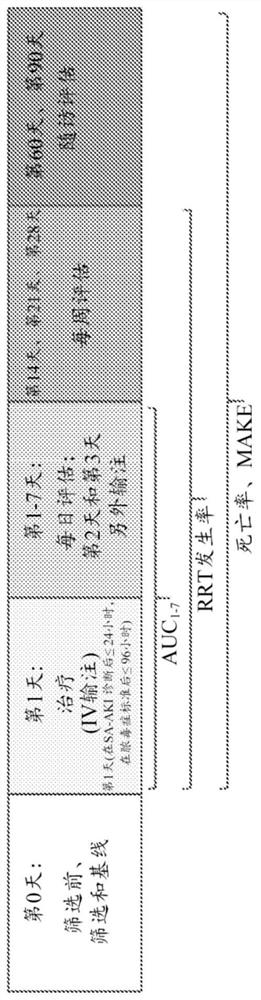
[0275] In part 1 of the adaptive phase 2a / 2b STOP-AKI trial, patients were randomly assigned to receive RecAP 0.4, 0.8, or 1.6 mg / kg or placebo once daily for 3 days to determine the optimal dose.
[0276] In part 2, this dose is compared to a placebo. The primary endpoint was the time-adjusted area under the endogenous creatinine clearance curve (AUC) from days 1 to 7. 1-7 ECC), with renal replacement therapy (RRT) as a key secondary endpoint. Long-term renal function, major adverse renal events (MAKE) at days 28, 60, and 9...
Embodiment 2
[0435] Determination of RecAP enzyme activity and protein concentration
[0436] Activity assay:
[0437] The determination of recAP enzyme activity is based on the conversion (hydrolysis) of 4-nitrophenol phosphate to yellow 4-nitrophenol. The change in optical density at 405 nm per unit time is a measure of alkaline phosphatase activity. The assay buffer consists of 0.25M glycine buffer (pH 9.6) and 2mM MgCl at 25°C 2 and 0.1mM ZnCl 2 and 8.5 mM 4-nitrophenol phosphate.
[0438] The unit (U) definition for recAP (expressed as U / mL) is the amount of enzyme that causes the hydrolysis of 1 μmol of 4-nitrophenol phosphate per minute at pH 9.6 and 25°C.
[0439] Protein concentration:
[0440] Determination of total protein concentration in RecAP drug substances and drug products is performed by UV / Vis analysis. RecAP solutions were analyzed at 280 nm and absorbance was a measure for protein content (mg / mL) using the following formula:
[0441] Concentration (mg / mL) = [A / (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com