Application of phospholipid polyethylene glycol modified subminiature cerium oxide nano-enzyme in preparation of medicine for treating acute kidney injury
An acute kidney injury and polyethylene glycol technology, applied in the application field of nano-biological materials, can solve the problems of increased ROS content in the kidney, acute kidney injury, etc., to prolong systemic circulation time, inhibit cell apoptosis, and have high biological safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Synthesis of ultra-small cerium oxide nanozyme modified with phospholipid polyethylene glycol
[0051] (1) Synthesis of ultra-small cerium oxide nanozymes:
[0052] Add 0.4g of cerium acetate hydrate and 3.2g of oleylamine to 15ml of xylene, raise the temperature to 90 degrees Celsius at a rate of 2 degrees Celsius per minute, and stir for 4 hours to form a complex; inject 1ml of deionized water into an inert gas In the protection reaction system, aging for three hours, precipitation with anhydrous ether, and centrifugation to obtain ultra-small cerium oxide nanozyme.
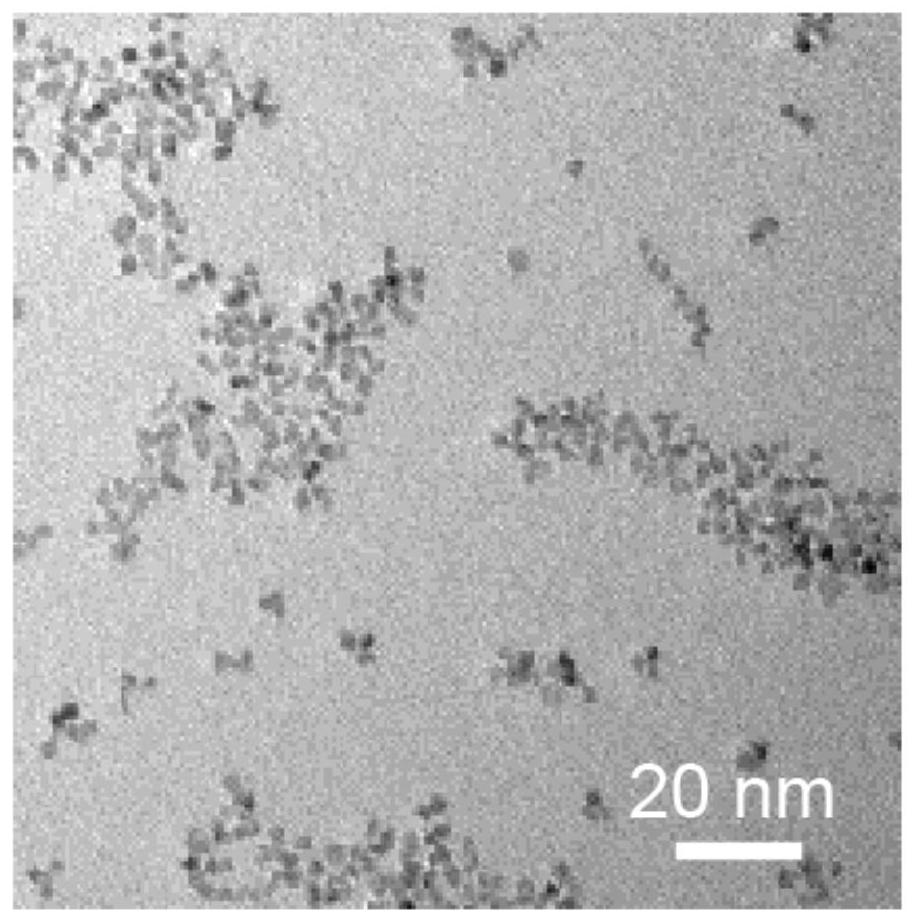
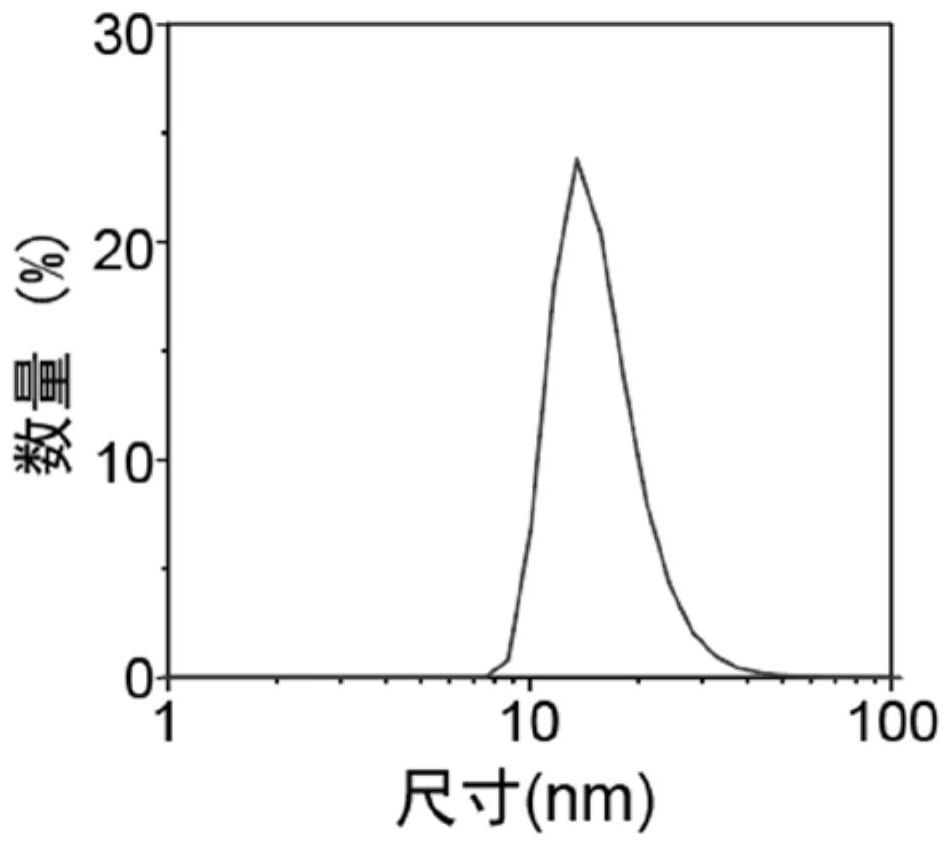
[0053] The result is as figure 1 As shown, the ultra-small cerium oxide nanozyme was characterized by transmission electron microscopy, and the particle size was about 1-10 nm.
[0054] (2) Synthesis of ultra-small cerium oxide nanozyme modified with phospholipid polyethylene glycol:
[0055] Add 0.01 g of polyethylene glycol phosphate and 1 ml of ultra-small cerium oxide nanozyme to 5 ml o...
Embodiment 2
[0058] Example 2: Effect of Cerium Oxide Nanozyme on HK-2 Damage Caused by Cisplatin
[0059] Preparation of the drug: the phospholipid polyethylene glycol-modified ultra-small cerium oxide nanozyme prepared in Example 1 was selected and dispersed in an aqueous solution to obtain a final ultra-small cerium oxide nanozyme concentration of 8.3 mg / ml.
[0060] Cell model establishment: add cisplatin-containing cell culture medium to HK-2 cells, and redisperse with cell culture medium to obtain the drug at a concentration of 200 mM.
[0061] Group settings:
[0062] a. Control group: normal cells were given fresh culture medium.
[0063] b. Model group: only cell culture solution containing cisplatin was added to HK-2 cells to make the final concentration 10 μM.
[0064]c. Treatment group: Add 200mM cisplatin to HK-2 cells and add cerium oxide with different concentration gradients to the cell culture medium, and make the final concentrations of cerium oxide 0.78μM, 1.56μM, 3.13...
Embodiment 3
[0066] Example 3: Effect of cerium oxide nanozyme on cisplatin-induced acute kidney injury
[0067] 20-22g male ICR mice were taken and induced with cisplatin to establish AKI model. First, the mice were randomly divided into 4 groups, blank control group, cisplatin model group, cisplatin model group + cerium oxide 0.5m / kg administration group, cisplatin model group + cerium oxide 1.5m / kg administration group, each group 6 only. Intraperitoneal injection of 15mg / kg cisplatin, while tail vein injection of 0.5mg / kg or 1.5mg / kg cerium oxide nanozyme. On the third day of administration, the rats were dissected, and blood was collected from the eyeballs to collect serum. After dissecting, the heart was perfused, and the kidneys of the mice were removed for paraffin embedding, HE staining and TUNEL staining.
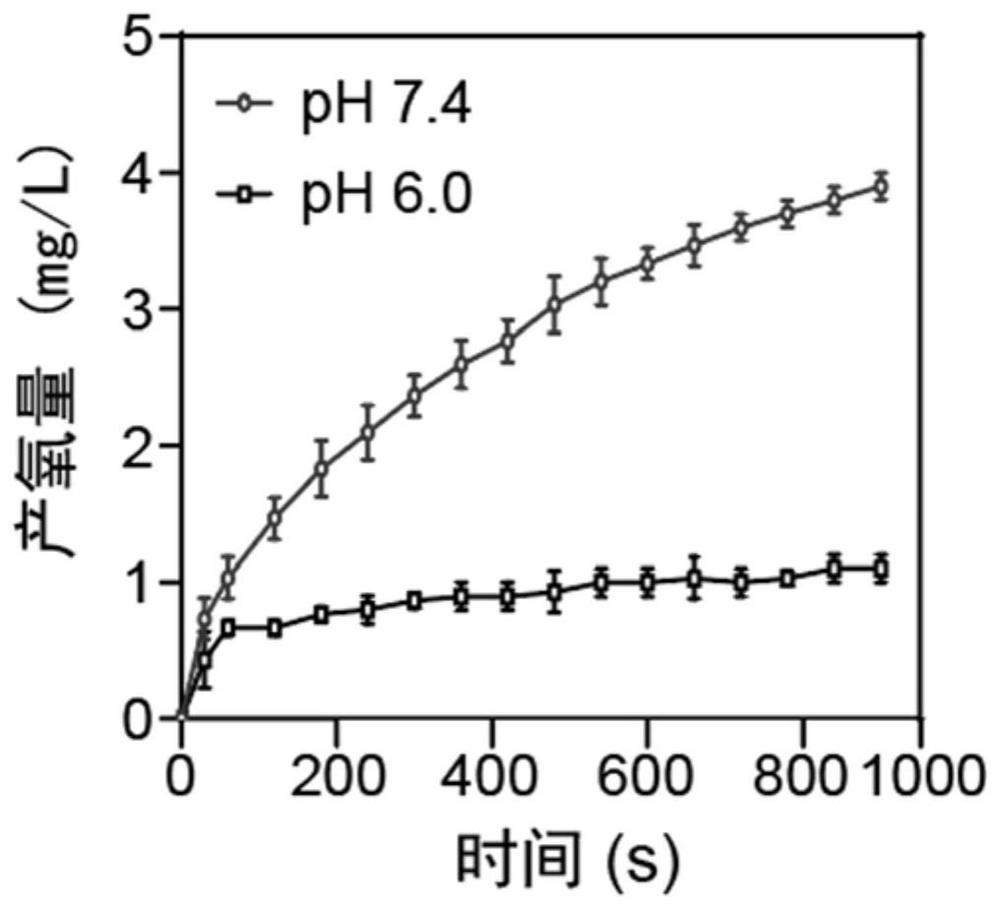
[0068] The result is as Figure 5 and Figure 6 It has been shown that cisplatin induces a significant increase in serum urea nitrogen and creatinine and increases in rena...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com