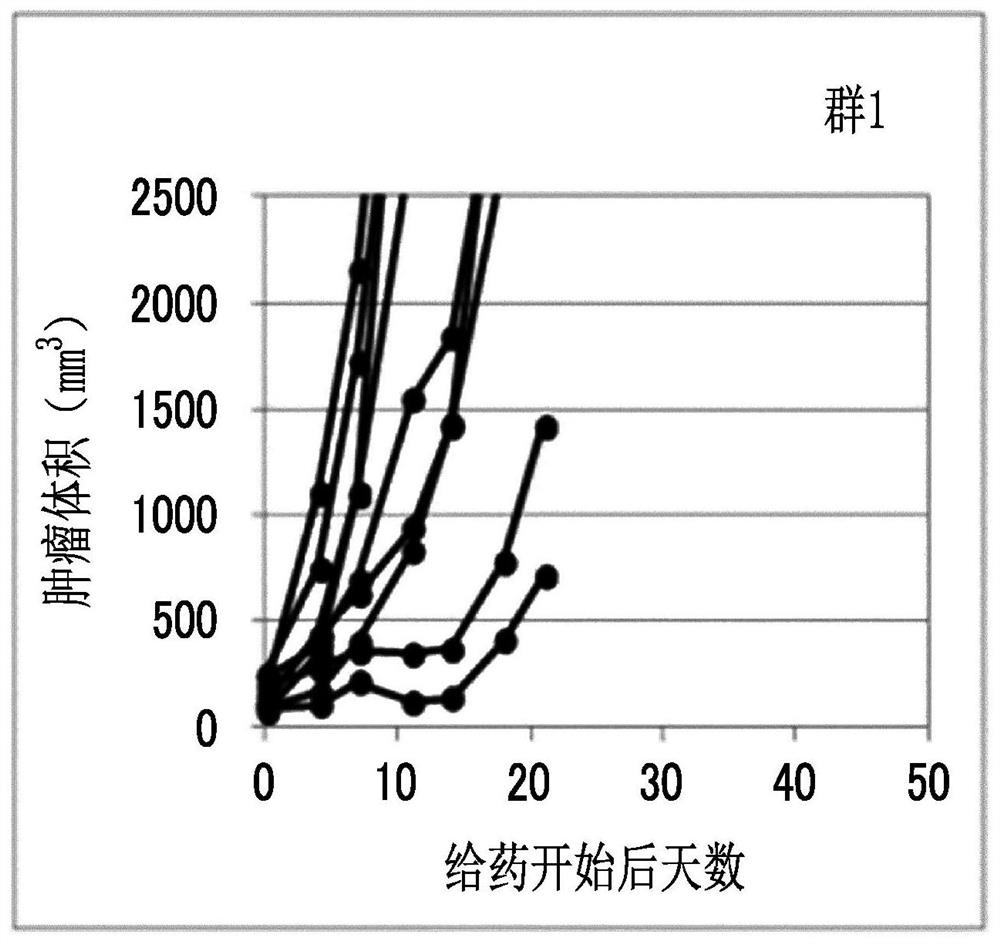
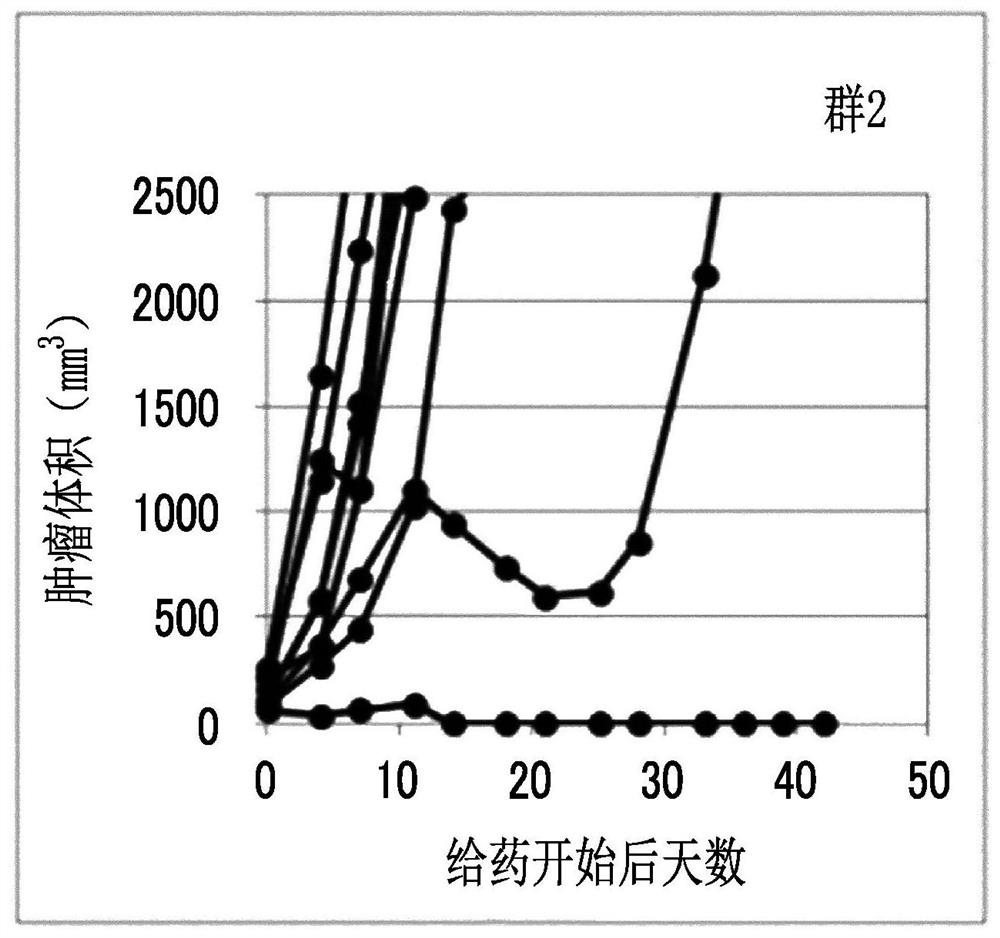
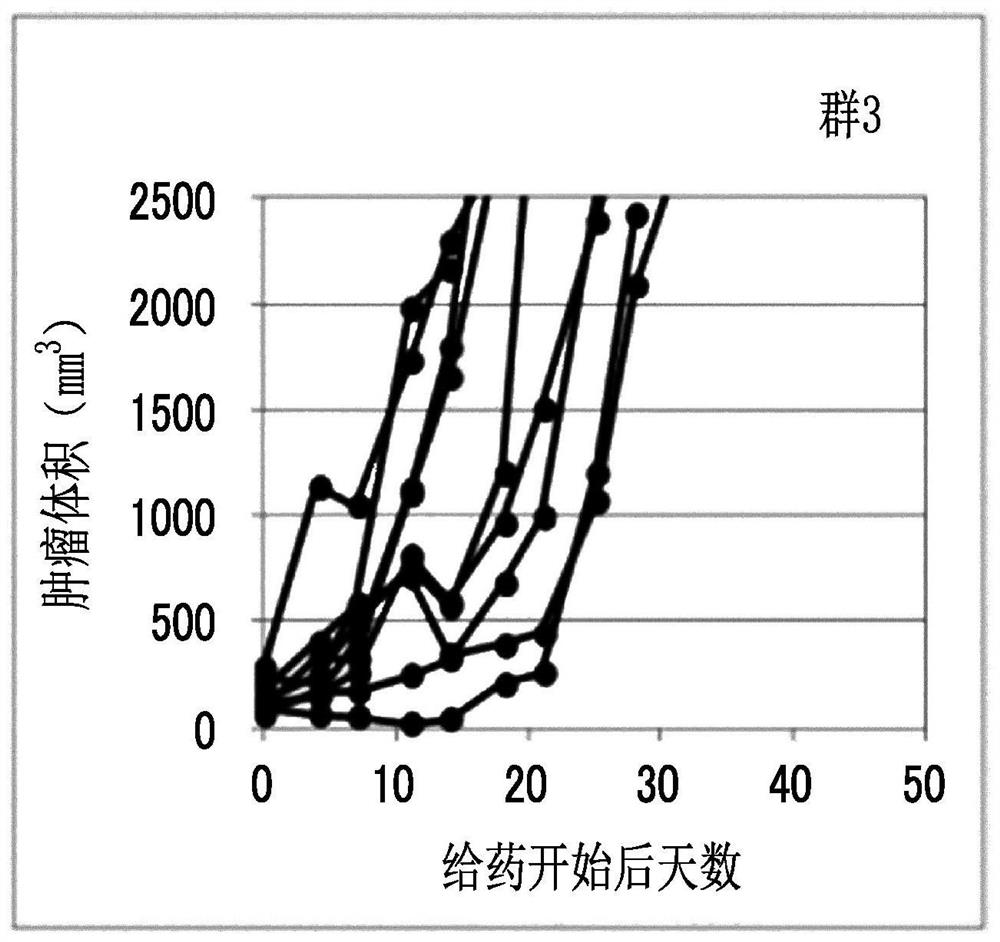
Combination medication containing liposome composition encapsulating drug and immune checkpoint inhibitor
A technology of immune checkpoint and liposome, which is applied in drug combination, liposome delivery, medical preparations containing active ingredients, etc., can solve the problems of insufficient improvement, and achieve less burden on the body, high half-life, strong Effect of antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0145] (b) preparation of the aqueous phase;
[0146] (c) liposome particle formation based on emulsification;
[0147] (d) extruder-based sizing;
[0148] (e) dialysis-based replacement of the liposome extracellular aqueous phase;
[0149] (f) entrapping the drug in liposome particles based on remote loading; and
[0150] (g) Dialysis-based removal of drugs from the external aqueous phase.
[0151] (d) Extruder-based sizing may or may not be performed.
[0152]
[0153] In (a) preparation of the oil phase, the components constituting the liposome (diacylphosphatidylethanolamine modified with a hydrophilic polymer, dihydrosphingomyelin, and cholesterol) and an organic solvent are mixed, and the mixture is subjected to The above-mentioned components are dissolved by raising the temperature, so that an oil phase can be produced.
[0154] The organic solvent used in the oil phase is not particularly limited, but for example, a water-soluble organic solvent that can be mixe...
Embodiment
[0221] Hereinafter, although an Example is given and this invention is demonstrated concretely, this invention is not limited to these at all. It is understood that the present invention can be variously changed or modified by those skilled in the art. Such changes and modifications are included in the present invention as long as they do not depart from the scope of the present invention. Commercial items were used for various reagents used in Examples unless otherwise specified.
[0222] SM represents Sphingomyelin (COATSOME NM-10, manufactured by NOF CORPORATION).
[0223] Egg-derived DHSM means dihydrosphingomyelin obtained by hydrogenating egg-derived SM (a synthetic product obtained by hydrogenating COATSOME NM-10 (manufactured by NOF CORPORATION)). This egg-derived DHSM has 2 alkyl chains having 16 carbon atoms in 70 to 80% of the whole, and the remainder contains a mixture of DHSMs having different alkyl chain lengths.
[0224] The total synthetic DHSM means dihydro...
Embodiment 1~8
[0260] (a) Preparation of oil phase
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com