Kynurenine Pathway Inhibitors
A technology for selecting compounds, applied in the field of application in the preparation of drugs for treating tumors, and can solve the problems of large dosage, many times of administration, and short half-life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
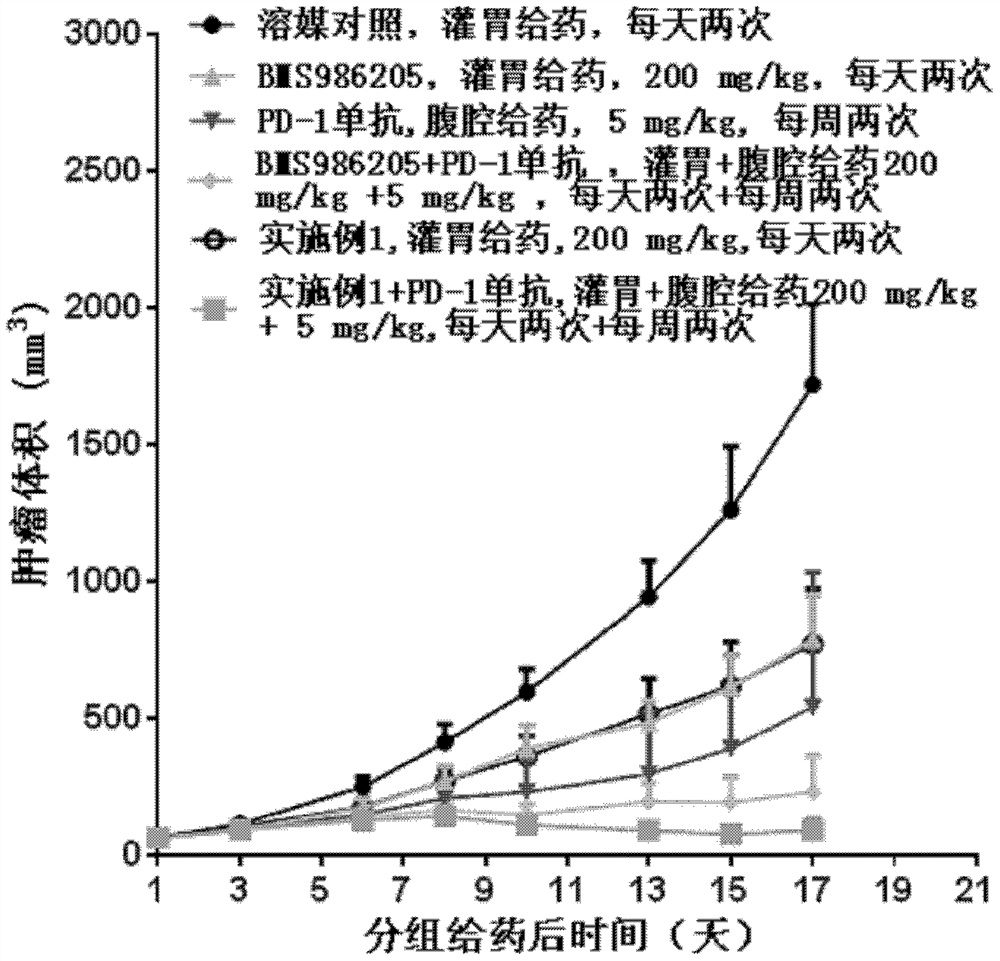
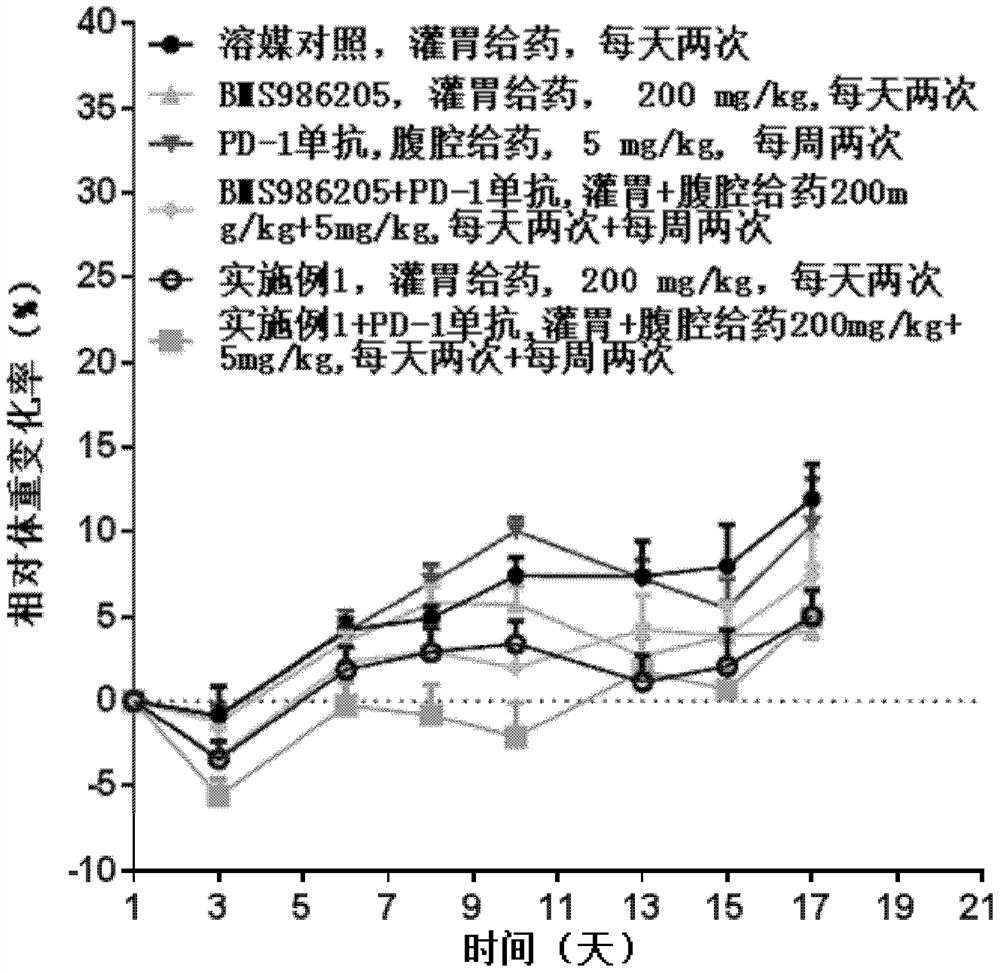
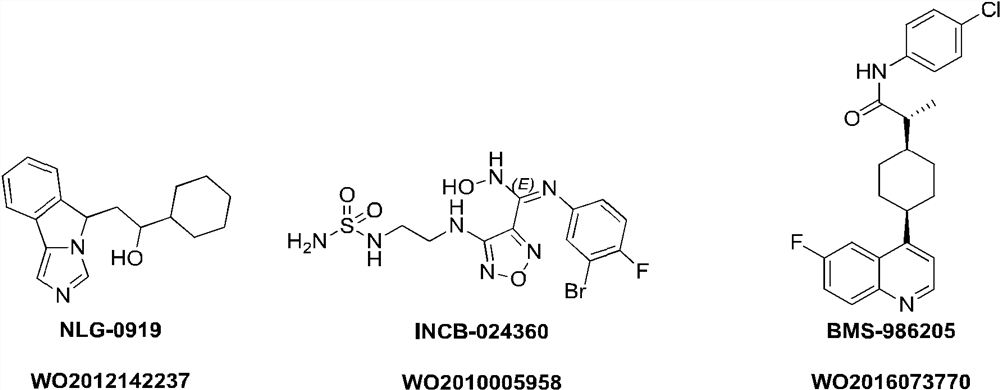
Image
Examples
Embodiment 1
[0119]
[0120]
[0121] Synthesis of compound 1-b:
[0122] At 0°C, iodomethane (185.33g) was added to compound 1-a (98.5g) in acetone (1500mL), reacted at 25°C for 1 hour, filtered, and the filter cake was spin-dried to obtain compound 1-b.
[0123] Synthesis of compound 1-d:
[0124] At 0°C, slowly drop thionyl chloride (96.92g, 814.69mmol) into absolute ethanol solution (1000mL), then add compound 1-c (95g, hydrochloride) and react at 80°C After 12 hours, it was concentrated under reduced pressure to obtain compound 1-d.
[0125] Synthesis of compound 1-e:
[0126] At 90°C, to a solution of compound 1-d (98g, hydrochloride) and N,N-diisopropylethylamine (141.01g, 1.09mol) in dioxane (1400mL) and water (350mL) Add compound 1-b (208.74g, 818.28mmol) in batches, react at 900°C for 12 hours, concentrate under reduced pressure to remove the solvent dioxane, add 1000 ml of ethyl acetate, and wash the solution with saturated sodium chloride solution for 3 times, 1000 ml...
Embodiment 2
[0141]
[0142] Synthesis of compound 2
[0143] Compound 1-l (50 mg) was dissolved in DMF (5 mL), and DIEA (98.39 mg) and HATU (86.84 mg) were added to the solution at 25° C. The resulting mixture was stirred at 25° C. for 30 minutes. Then, 2-a (23.59 mg) was added to the reaction solution, and the resulting mixture was stirred at 25°C for 12 hours. Water (10 mL) was added to the reaction solution to quench the reaction and extracted with ethyl acetate (30 mL*3). The combined organic phases were washed with Na 2 SO 4 Dry, filter and concentrate under reduced pressure to obtain the crude product. The crude product is purified by preparative chromatography to obtain compound 2.
[0144] 1 H NMR (400MHz, CCl 3 D) δppm 1.69-1.91 (m, 3H) 2.00-2.10 (m, 3H) 2.33-2.56 (m, 6H) 3.00 (br d, J = 11.74Hz, 2H) 3.18 (br t, J = 11.80Hz, 1H )6.77-6.88 (m, 2H) 7.31 (d, J = 4.65Hz, 1H) 7.39-7.48 (m, 1H) 7.61 (dd, J = 10.33, 2.63Hz, 1H) 8.03-8.15 (m, 1H) 8.28 (td, J=9.23, 5.99Hz, 1H) 8.8...
Embodiment 3
[0146]
[0147] Synthesis of Compound 3
[0148] Compound 1-l (50 mg) was dissolved in DMF (3 mL), and DIEA (98.39 mg) and HATU (86.84 mg) were added to the solution at 25° C. The resulting mixture was stirred at 25° C. for 30 minutes. Then, to 3-a (23.31 mg) was added to the reaction solution, and the resulting mixture was stirred at 25°C for 12 hours. Water (10 mL) was added to the reaction solution to quench the reaction and extracted with ethyl acetate (20 mL*3). The combined organic phases were washed with Na 2 SO 4 Dry, filter and concentrate under reduced pressure to obtain the crude product. The crude product is purified by preparative chromatography to obtain compound 3.
[0149] 1 H NMR (400MHz, CCl 3 D) δppm 1.68-1.92 (m, 3H) 1.96-2.10 (m, 3H) 2.25-2.58 (m, 6H) 3.02 (br d, J = 11.25Hz, 2H) 3.18 (br t, J = 12.10Hz, 1H ) 7.01 (dd, J = 8.01, 1.04Hz, 1H) 7.17 (s, 1H) 7.21 (s, 1H) 7.37-7.48 (m, 2H) 7.60 (dd, J = 10.33, 2.63Hz, 1H) 7.66 (s , 1H) 8.07-8.14 (m, 1H) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com