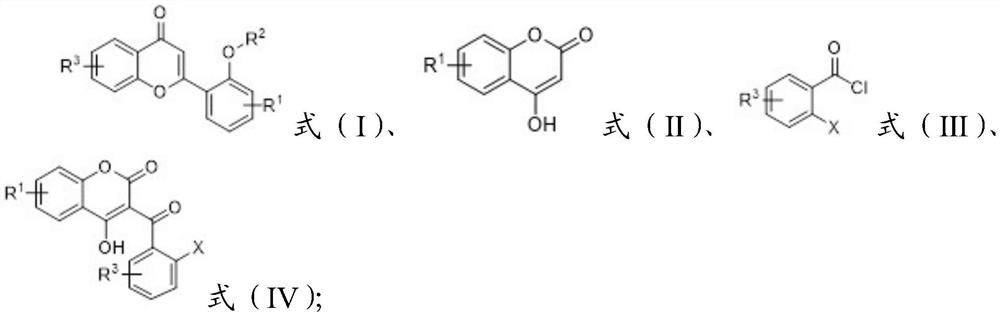
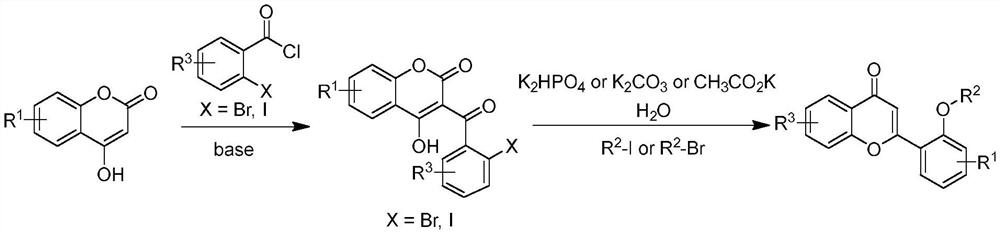
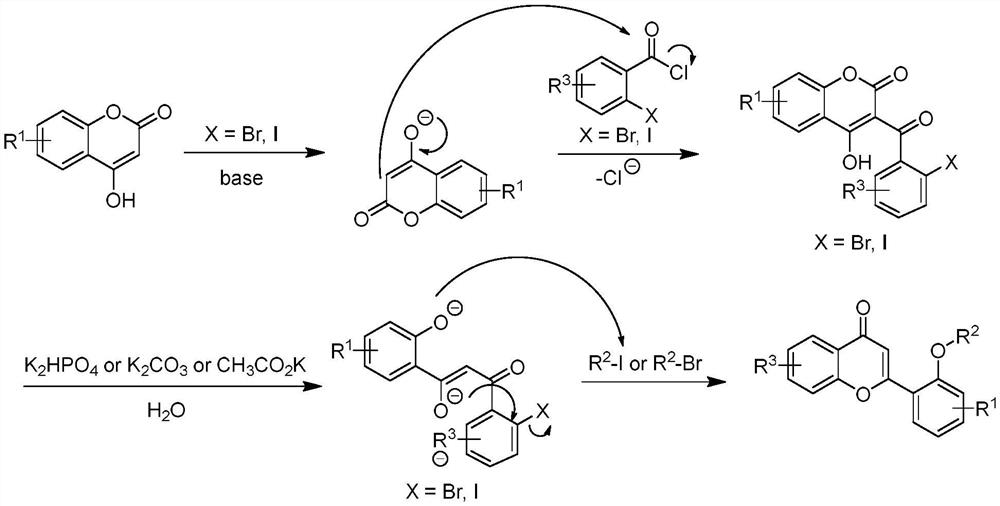
A long-lasting light-storage organic light-emitting material and its preparation method
A luminescent material and light-storage technology, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of low luminous brightness of luminescent materials, single color of luminescent materials, weak acid and alkali resistance, etc., and achieve the reaction route Novelty, low preparation cost and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] This embodiment is the preparation of 2-(2-methoxyphenyl)-benzopyran-4-one, and the specific preparation steps are as follows:
[0033]
[0034] 1) At room temperature, add 1.0 equivalents of 4-hydroxycoumarin to DCM, slowly add 1.2 equivalents of pyridine during the stirring process, wait until the base is added, stir at a rate of 1000r / min, stir for 1.5h, wait until the temperature Cool down to room temperature, add 2.0 equivalents of 2-iodobenzoyl chloride, and react for 9h. After the reaction was completed, the reaction system was slowly added dropwise to ice water, extracted three times with ethyl acetate, the organic phases were combined, extracted once with dilute hydrochloric acid, distilled water, and saturated saline, dried over anhydrous magnesium sulfate, filtered, and rotary evaporated. The product 3-(2-iodobenzoyl)-4-hydroxycoumarin was washed and purified with a mixture of petroleum ether and absolute ethanol at a volume ratio of 2:1, a white solid wit...
Embodiment 2
[0037] The difference between this example and Example 1 is that the methyl iodide in step 2 is replaced by methyl bromide. After testing, step 1) successfully obtained 3-(2-iodobenzoyl)-4-hydroxycoumarin with a yield of 84%, and step 2) successfully obtained 2-(2-methoxyphenyl)-benzo Pyran-4-one, 76% yield.
Embodiment 3
[0039] The difference between this example and Example 1 is that in step 1), there are 1.0 equivalents of 4-hydroxycoumarin, 2.0 equivalents of pyridine, and 2.0 equivalents of 2-iodobenzoyl chloride. After testing, step 1) successfully obtained 3-(2-iodobenzoyl)-4-hydroxycoumarin with a yield of 78%, and step 2) successfully obtained 2-(2-methoxyphenyl)-benzo Pyran-4-one, 74% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com