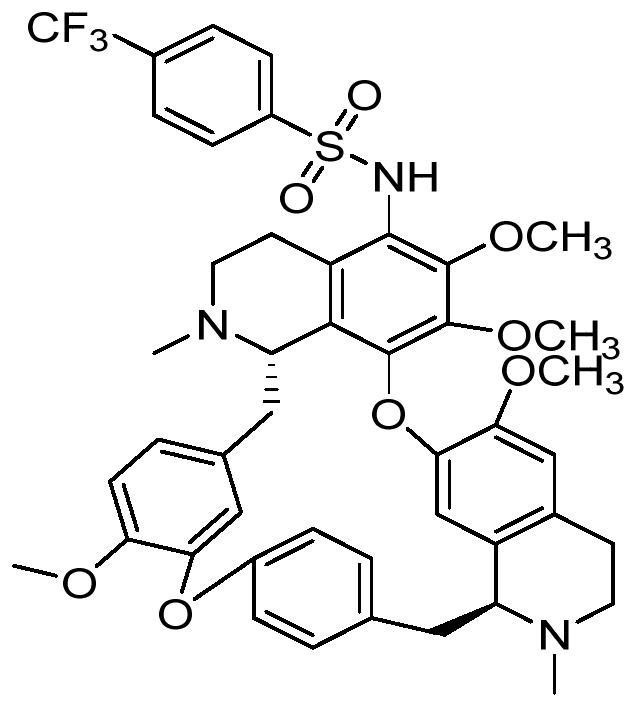
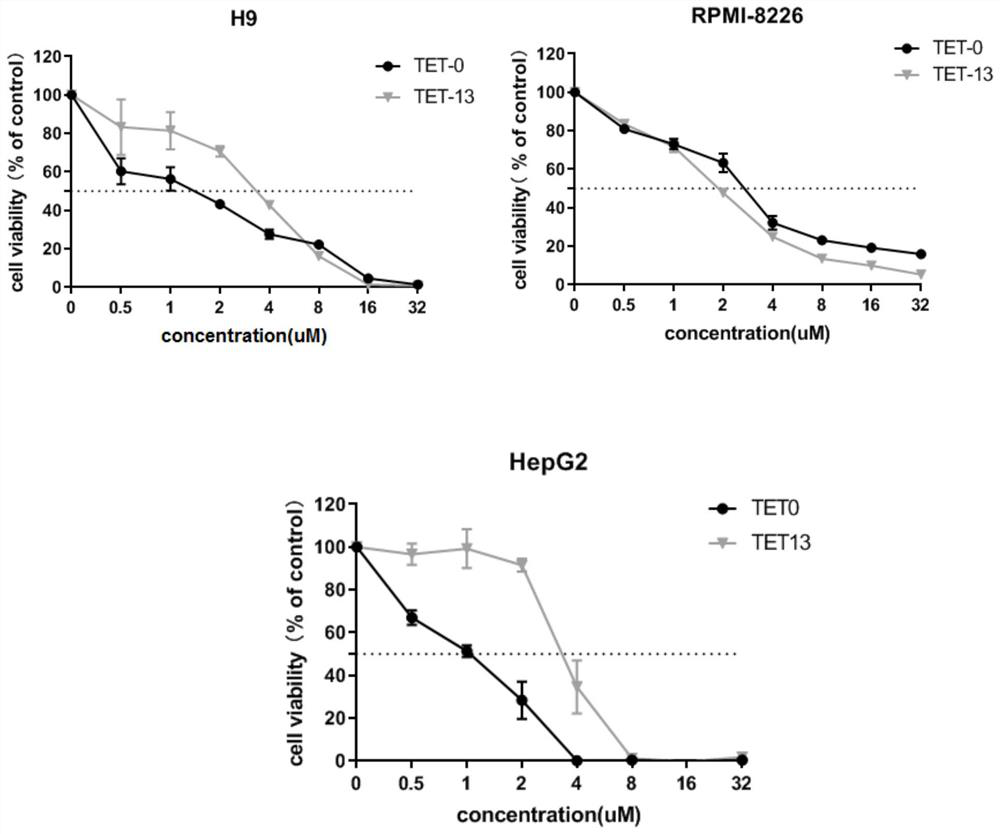
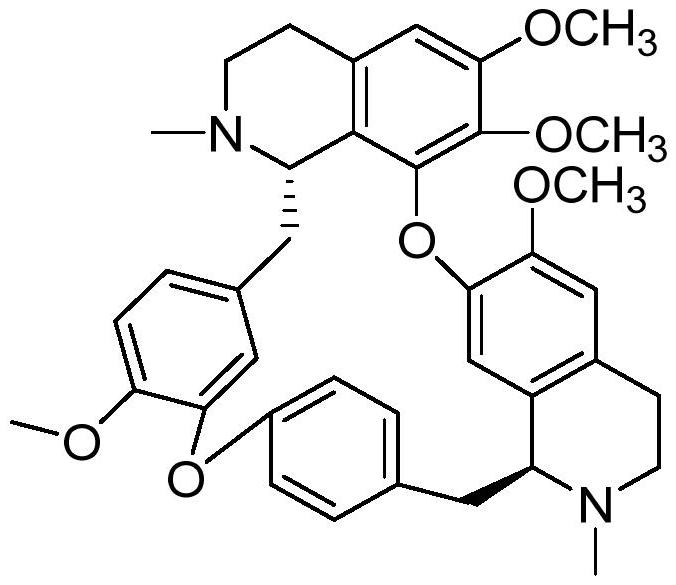
C5-position substituted tetrandrine derivatives and preparation method and application thereof
A technology of tetrandrine and bromidentine, which is applied in the field of preparation of tetrandrine derivatives substituted at the C5-position, can solve the problem of little change in pharmacokinetic parameters and achieve obvious anti-cell line activity , good biological activity, and the effect of improving the activity of cell lines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0031] 5-bromine anti-hexametin prepare: Han anti-hexametin is dissolved in glacial acetic acid, add tribrompyridium salt, reaction at room temperature for a certain time, reaction liquid nodide, combined with dichloromethane, combined with saline , Dry sodium sulfate, dry, to give 5-bromoan anti-hexarin. 1 H NMR (400MHz, CDC1 3), Δ7.34 (DD, J = 8.2, 2.2 Hz, 1H), 7.14 (DD, J = 8.1, 2.5 Hz, 1H), 6.87 (S, 2H), 6.79 (DD, J = 8.3, 2.5 Hz, 1H), 6.52 (S, 1H), 6.50 (S, 1H), 6.29 (DD, J = 8.3, 2.1 Hz, 1H), 6.01 (S, 1H), 3.93 (S, 3H), 3.79 (D, J = 10.1 Hz, 1H), 3.74 (S, 3H), 3.55-3.40 (m, 3H), 3.26 (S, 3H), 3.26 (DD, J = 12.5, 5.6Hz, 1H), 3.22 (S, 3H) 3.02-2.95 (m, 2H), 2.91-2.87 (m, 1H), 2.82-2.67 (m, 5H), 2.64 (S, 3H), 2.49 (D, J = 8.4 Hz, 1H), 2.29 (S , 3H); ESI-MS M / Z: 701.4 [M + H] + .
[0032]
[0033] Preparation of 5- Cyanyl Han anti-hexametin (TET-01): Take a proper amount of 5-bromo-anthropide in diendylformamide solution, add cyanide, allowed to heat insulation at 140-150 ° ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com