C5-substituted tetrandrine derivative as well as preparation method and application thereof
A technology of tetrandrine and its derivatives, which is applied in the field of natural medicine and medicinal chemistry, and can solve the problem of little change in pharmacokinetic parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
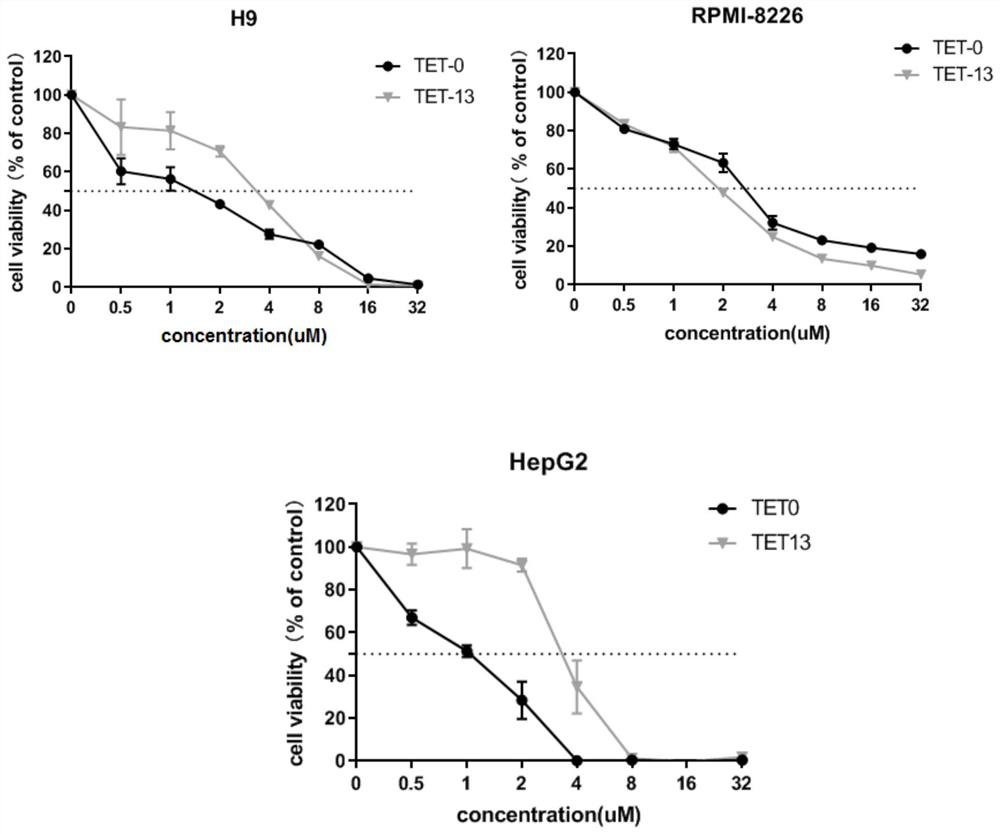
Examples
Embodiment
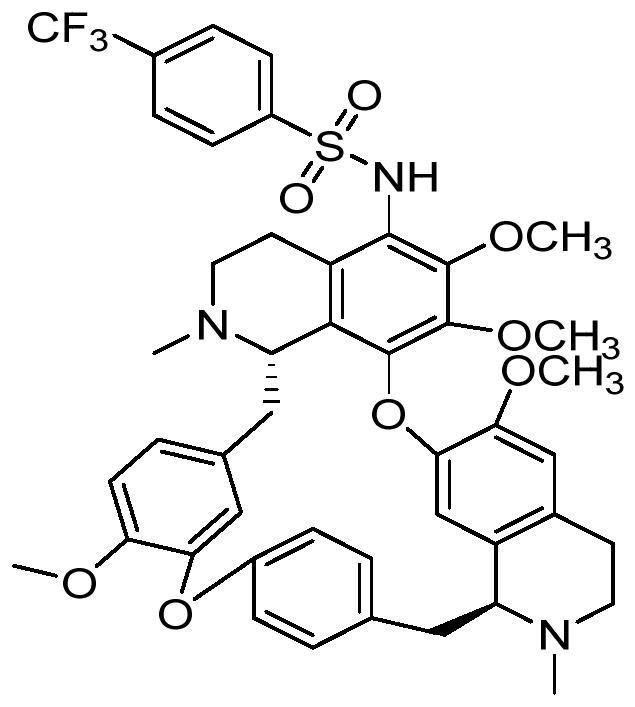
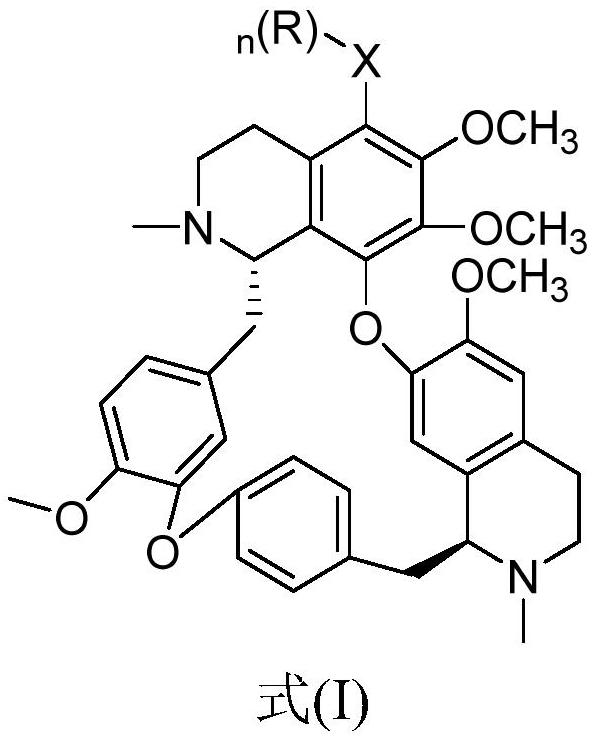
[0031] Preparation of 5-bromotetrandrine: Dissolve tetrandrine in glacial acetic acid, add tribromopyridinium salt, react for a certain period of time at room temperature, adjust the base of the reaction solution and extract it with dichloromethane, combine the organic layers, and wash with brine , dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 5-bromotetrandrine. 1 H NMR (400MHz, CDC1 3), δ7.34(dd, J=8.2, 2.2Hz, 1H), 7.14(dd, J=8.1, 2.5Hz, 1H), 6.87(s, 2H), 6.79(dd, J=8.3, 2.5Hz, 1H),6.52(s,1H),6.50(s,1H),6.29(dd,J=8.3,2.1Hz,1H),6.01(s,1H),3.93(s,3H),3.79(d,J =10.1Hz,1H),3.74(s,3H),3.55-3.40(m,3H),3.37(s,3H),3.26(dd,J=12.5,5.6Hz,1H),3.22(s,3H) ,3.02-2.95(m,2H),2.91-2.87(m,1H),2.82-2.67(m,5H),2.64(s,3H),2.49(d,J=8.4Hz,1H),2.29(s ,3H); ESI-MS m / z:701.4[M+H] + .
[0032]
[0033] Preparation of 5-cyanotetrandrine (TET-01): Dissolve an appropriate amount of 5-bromotetrandrine in diethylformamide solution, add cuprous cyanide, and keep it warm at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com