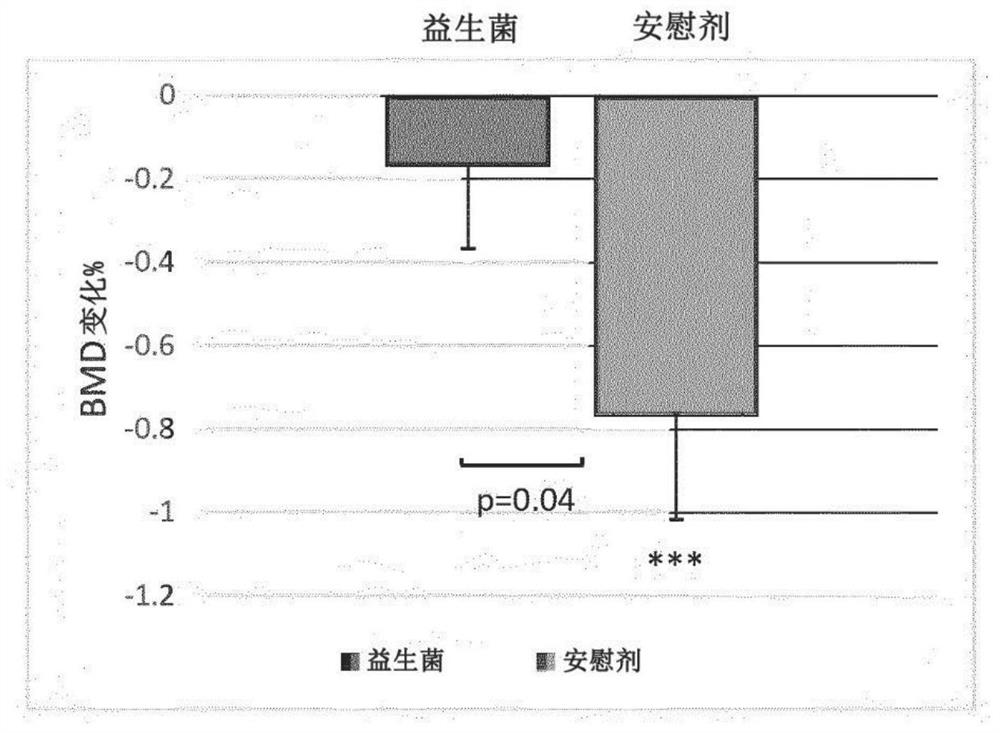
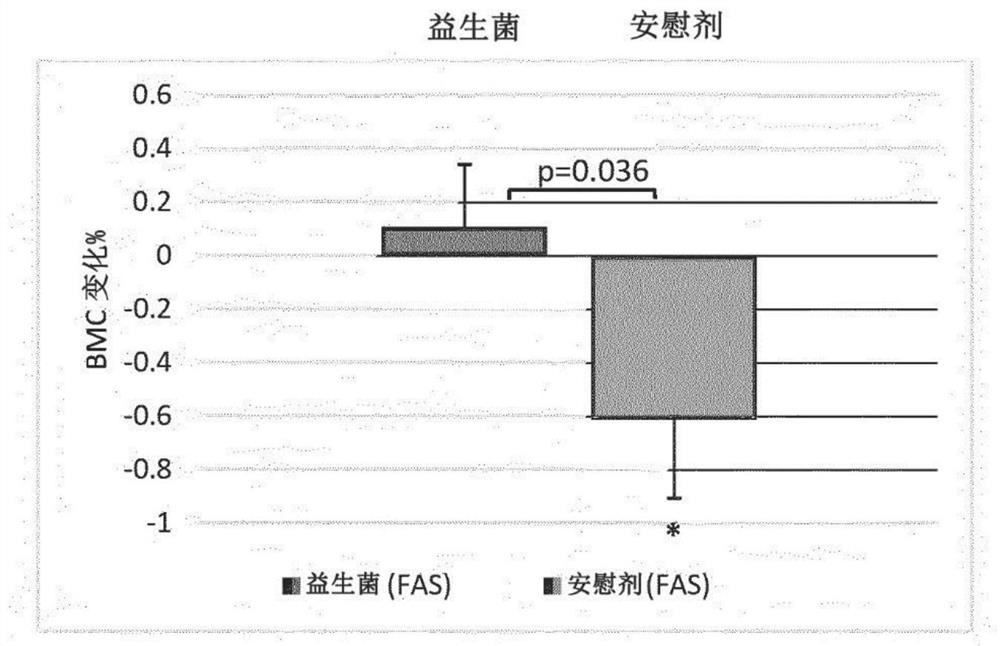
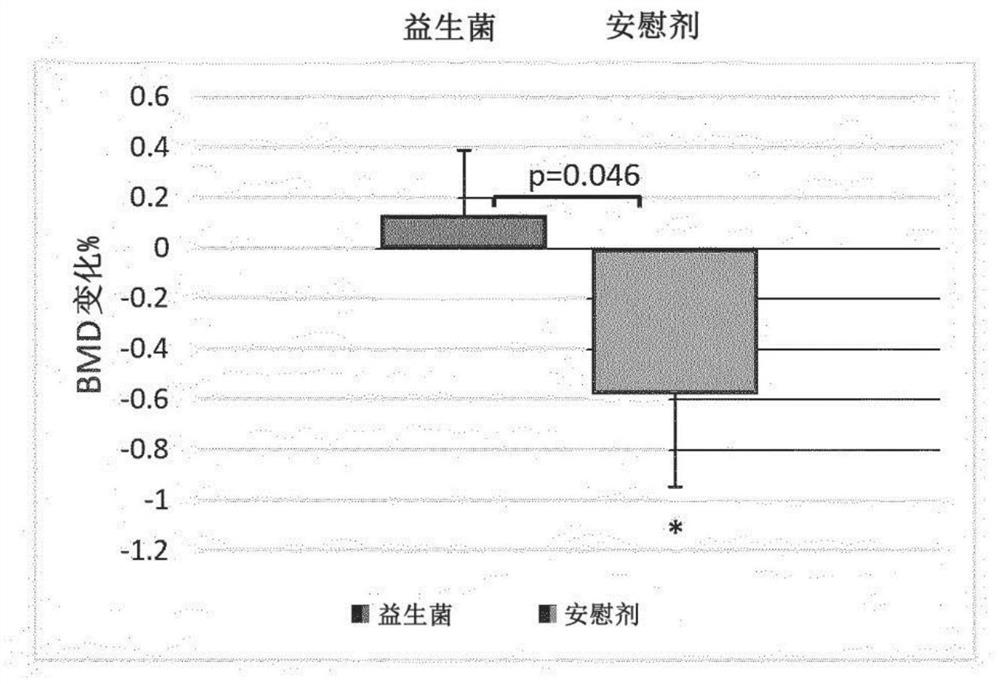
Probiotic compositions and uses thereof
A technology of probiotics and compositions, applied in the field of probiotics strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example A
[0118] Example A: Tablet
[0119] Probiotic strains 1x10 9 CFU or preferably 1x10 10 CFU
[0120]
[0121] Tablets are prepared from the above ingredients by wet granulation followed by compression.
example B
[0122] Example B: Tablet Formulation
[0123] Formulations A and B below were prepared by wet granulating the ingredients with a solution of povidone, then adding magnesium stearate and by compression.
[0124] Formulation A
[0125]
[0126] (* = or preferably 1x10 10 CFU)
[0127] Formulation B
[0128]
[0129] (* = or preferably 1x10 10 CFU)
[0130] Formulation C
[0131]
[0132] Formulations D and E below were prepared by direct compression blending the ingredients. The lactose used in Formulation E is of the direction compression type.
[0133] Formulation D
[0134] Probiotic strains 1x10 9 CFU or preferably 1x10 10 CFU
[0135] Pregelatinized Starch NF15 150mg
[0136] Formulation E
[0137] Probiotic strains 1x10 9 CFU or preferably 1x10 10 CFU
[0138] Lactose 150mg
[0139] 100mg
[0140] Formulation F (controlled release formulation)
[0141] The formulation was prepared by wet granulating the ingredients (below) with a ...
example C
[0144] Example C: Capsule formulation
[0145] Formulation A
[0146] A capsule formulation was prepared by mixing the ingredients of Formulation D in Example B above and filling into two-section hard gelatin capsules. Formulation B (below) was prepared in a similar manner.
[0147] Formulation B
[0148]
[0149] Formulation C
[0150] (a) Probiotic strains 1x10 9 CFU or preferably 1x10 10 CFU
[0151] (b) Macrogol 4000BP 350mg
[0152] Capsules were prepared by: melting polyethylene glycol 4000BP; dispersing one or more probiotic strains in the melt; and filling the melt into two-section hard gelatin capsules.
[0153] Formulation D (controlled release capsule )
[0154] The following controlled release capsule formulation was prepared by extruding ingredients a, b and c using an extruder, then spheronizing the extrudate and drying. The dried granules were then coated with a release controlling film (d) and filled into two-part hard gelatin capsules.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com