Human-derived Anti-(poly-ga) dipeptide repeat (DPR) antibody
A repetitive sequence, VH-CDR2 technology, applied in the direction of antibodies, dipeptides, radioactive carriers, etc., can solve the potential immunogenic properties of inherently unstable compounds in RNA, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0600] Example 1: Isolation and identification of anti-(poly-GA) dipeptide repeat (DPR) protein antibodies
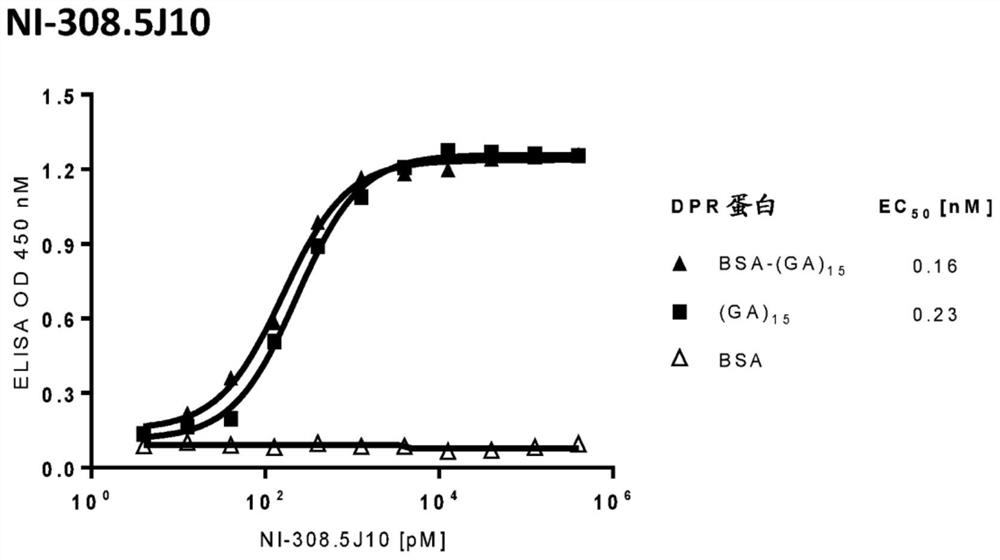
[0601] Human antibodies targeting poly-GA dipeptide repeat (DPR) proteins, fragments thereof, C9orf72-DPR and / or fragments thereof were identified based on the methods described in international application WO 2016 / 050822 A2, which The disclosure of is incorporated herein by reference. In particular, the poly-GA dipeptide repeat protein GA was synthesized and purified by Schafer-N (Copenhagen, Denmark) 15 : H-CHHHHHH(GA) 15 -OH) (SEQ ID NO: 61). The poly-GA dipeptide repeat protein was then conjugated to bovine serum albumin (BSA) via a bifunctional linker (SMCC). Subsequently, direct ELISA was performed directly using a 96-well microplate (Corning) in coating buffer (15 mM Na 2 CO 3 , 35mM NaHCO 3 Coated with non-conjugated or BSA-conjugated poly-GA dipeptide repeat protein or BSA (Sigma-Aldrich, Buchs, Switzerland) at a concentration of 5 μg / ml in , pH 9.42). A...
Embodiment 2
[0602] Example 2: Determination of antibody sequences
[0603] The amino acid sequences of the variable regions of the anti-(poly-GA)DPR antibodies identified above were determined based on their mRNA sequences, see figure 1 A-F. Briefly, live B cells of selected non-immortalized memory B cell cultures were harvested. Subsequently, mRNA was extracted from cells producing the selected anti-(poly-GA)DPR antibody and converted to cDNA, and the sequence encoding the variable region of the antibody was amplified by PCR and cloned into a plasmid vector and sequenced. Briefly, primer combinations representing all sequence families of the human immunoglobulin germline repertoire were used for amplification of leader peptides, V-segments and J-segments. A first round of amplification was performed using leader peptide specific primers at the 5' end and constant region specific primers at the 3' end (Smith et al., Nat Protoc. 4 (2009), 372-384). For heavy and kappa light chains, a ...
Embodiment 3
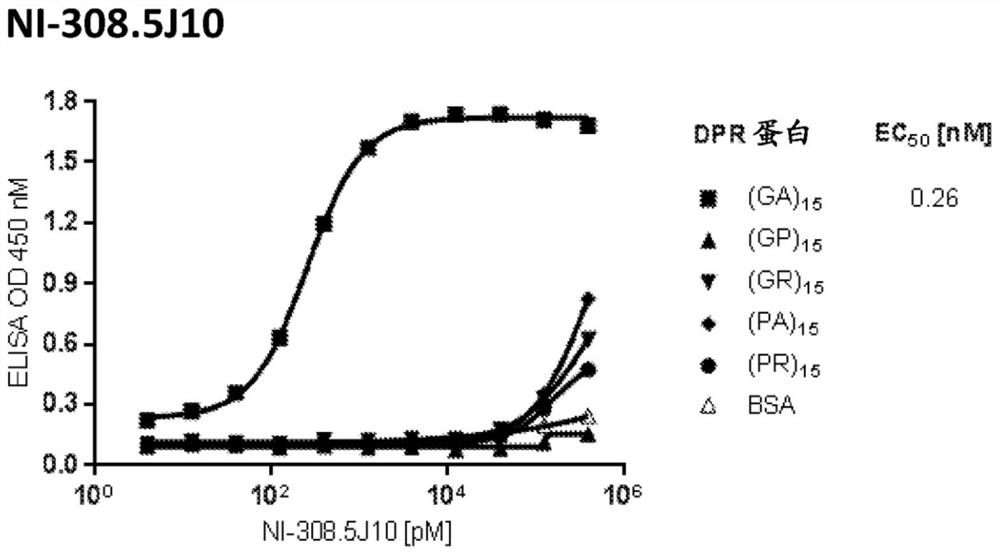
[0607] Example 3: ELISA EC for C9orf72 dipeptide repeat protein 50 analyze
[0608] In order to determine the binding specificity and half maximal effective concentration (EC) of recombinant human C9orf72 antibody NI-308.5J10 to C9orf72 poly-GA DPR 50 ), ELISA EC 50 analyze. Briefly, dipeptide repeat proteins were synthesized and purified by Schafer-N (Copenhagen, Denmark): (GA) 15 : H-CHHHHHH(GA) 15 -OH (SEQ ID NO: 61); (GP) 15 : H-C(GP) 15 -OH (SEQ ID NO: 62); (GR) 15 : H-C(GR) 15 -OH (SEQ ID NO: 63); (PA) 15 : H-C(PA) 15 -OH (SEQ ID NO: 64); (PR) 15 : H-C(PR) 15 -OH (SEQ ID NO: 65). A 96-well microplate (Corning Incorporated, Corning, USA) was coated with coating buffer (15 mM Na 2 CO 3 , 35mM NaHCO 3 , pH 9.42) at a concentration of 5 μg / ml or 20 μg / ml dipeptide repeat protein peptide coating. At room temperature with PBS / 0.1% containing 2% BSA (Sigma-Aldrich, Buchs, Switzerland) -20 to block non-specific binding sites for 1 hr. NI-308.5J10 was diluted t...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap