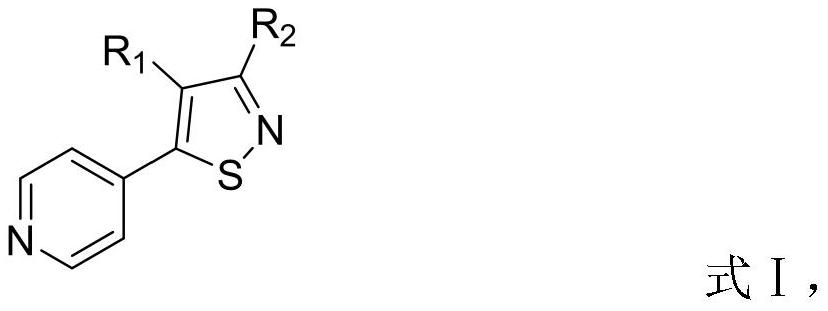
3-hydroxyisothiazole derivative as well as preparation method and application thereof
A technology for hydroxyisothiazole and derivatives, which is applied in the field of 3-hydroxyisothiazole derivatives and their preparation, can solve the problems of high toxicity to aquatic organisms, long synthesis routes, complicated processes, etc., and achieves low cost, simple preparation method steps, The effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
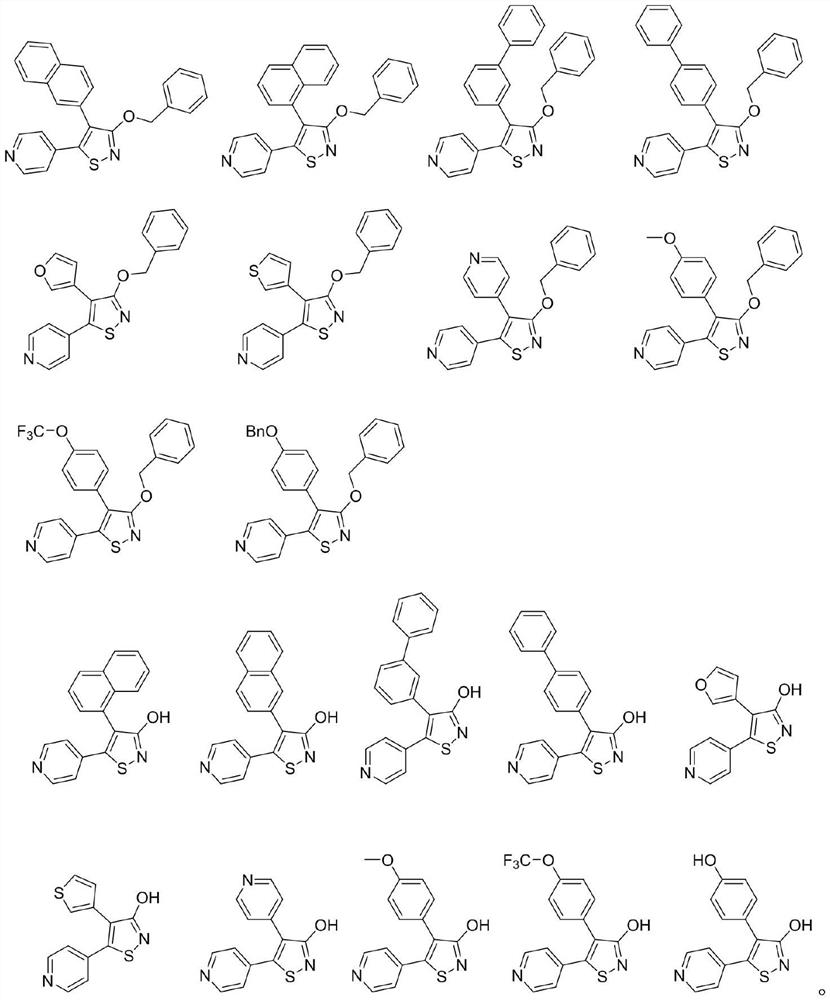
[0033] Preparation of 3-hydroxy-4-(2-naphthyl)-5-(4-pyridyl)-isothiazole (I-1)
[0034] The preparation of I-1 comprises the following three steps:
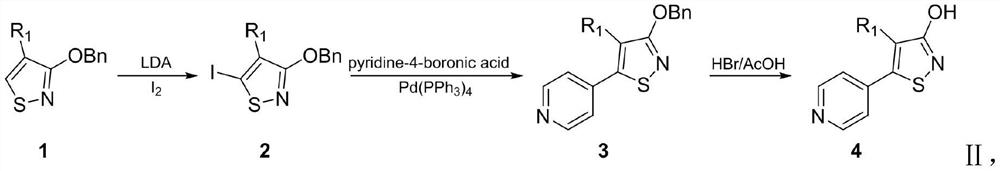
[0035] Step 1: Preparation of 3-benzyloxy-4-(2-naphthyl)-5-iodo-isothiazole
[0036] Add 0.66g (2.06mmol) 3-benzyloxy-4-(2-naphthyl)-isothiazole to a 50mL two-necked flask, then protect it with argon, and add 1.55mL (3.10mmol) LDA dropwise at -78°C , after stirring for 15 minutes, dissolve 0.63g (2.48mmol) iodine in 20mL tetrahydrofuran, slowly drop into the flask, and continue stirring for 30min. After the reaction was completed, 10 mL of water was added to quench, and a little solid sodium thiosulfate was added to stir for 2 h, then extracted with ethyl acetate (30 mL×3), dried over anhydrous sodium sulfate, and concentrated by filtration. Purified by column chromatography to obtain a white solid with a yield of 90.11%; the hydrogen spectrum test result of the product 1 H NMR (400MHz, CDCl 3 ): δ7.97-7.94 (m, 1H, ArH), 7.92...
Embodiment 2
[0041] Preparation of 3-hydroxy-4-(1-naphthyl)-5-(4-pyridyl)-isothiazole (I-2)
[0042] The preparation of I-2 comprises the following three steps:
[0043] Step 1: Preparation of 3-benzyloxy-4-(1-naphthyl)-5-iodo-isothiazole
[0044] Add 0.66g (2.06mmol) of 3-benzyloxy-4-(1-naphthyl)-isothiazole into a 50mL two-necked flask and protect it with argon, then add 1.55mL (3.10mmol) of LDA dropwise at -78°C After stirring for 15 minutes, dissolve 0.63g (2.48mmol) of iodine in 20mL of tetrahydrofuran, slowly drop it into the flask, and continue to stir for 30min. Extracted with ethyl acetate (30mL×3), dried over anhydrous sodium sulfate, concentrated by filtration, and purified by column chromatography to obtain a white solid with a yield of 88.65%; mp (melting point) 127-129°C; 1 HNMR (400MHz, CDCl 3 ): δ7.98-7.88(m, 2H, ArH), 7.61-7.35(m, 5H, ArH), 7.30-7.12(m, 5H, ArH), 5.37(s, 2H, CH 2 ); MS (ESI): m / z 443.95 (M+H) + .
[0045] Step 2: Preparation of 3-benzyloxy-4-(1-napht...
Embodiment 3
[0049] Preparation of 3-Hydroxy-4-(3-biphenyl)-5-(4-pyridyl)-isothiazole (I-3)
[0050] The preparation of I-3 comprises the following three steps:
[0051] Step 1: Preparation of 3-benzyloxy-4-(3-biphenyl)-5-iodo-isothiazole
[0052] After adding 0.71g (2.06mmol) of 3-benzyloxy-4-(3-biphenyl)-isothiazole into a 50mL two-necked flask and protecting it with argon, add 1.55mL (3.10mmol) dropwise at -78°C LDA, after stirring for 15 minutes, dissolve 0.63g (2.48mmol) iodine in 20mL tetrahydrofuran, slowly drop it into the flask, continue to stir for 30min, after the reaction is completed, add 10mL water to quench, add a little sodium thiosulfate solid and stir for 2h, It was extracted with ethyl acetate (30mL×3), dried over anhydrous sodium sulfate, concentrated by filtration, and purified by column chromatography to obtain a white solid with a yield of 87.34%; mp 105-107°C; 1 H NMR (400MHz, CDCl 3 )δ7.99-7.84(m, 5H), 7.59(d, J=1.6Hz, 1H), 7.57(d, J=1.6Hz, 1H), 7.55-7.46(m, 2H)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap