Anti-cd3 antibodies and uses thereof
An antibody and antigen technology, applied in the direction of antibodies, antibody medical components, anti-tumor drugs, etc., can solve problems such as unfavorable pharmacokinetics, immunogenicity and manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0206] Preparation of immunogenic antigens and production of monoclonal antibodies can be performed by any suitable technique such as recombinant protein production. The immunogenic antigen can be administered to the animal as a purified protein or protein mixture (including whole cells or cell or tissue extracts), or the antigen can be formed de novo in the animal from nucleic acid encoding the antigen or a portion thereof.
[0207] Generation and use of bispecific and multispecific CD3 antibodies
[0208] The present invention provides bispecific and multispecific antibodies comprising a first domain that specifically binds CD3 and a second domain that specifically binds a second antigen. The second antigen can be a tumor associated antigen (TAA) or an antigen on a pathogenic cell.
[0209] Exemplary anti-CD3 antibodies that can be used to engineer bispecific and multispecific antibodies comprising a first domain that specifically binds CD3 and a second domain that specif...
Embodiment approach
[0565] The present invention provides the following non-limiting embodiments.
[0566] 1. An isolated recombinant anti-CD3 antibody or antigen-binding fragment thereof, comprising:
[0567] a) a heavy chain and a light chain comprising: a heavy chain complementarity determining region (HCDR) 1 comprising SEQ ID NO:662, a HCDR2 comprising SEQ ID NO:663 and a HCDR3 comprising SEQ ID NO:664, wherein The light chain comprises: a light chain complementarity determining region (LCDR) 1 comprising SEQ ID NO: 671, an LCDR2 comprising SEQ ID NO: 673 and an LCDR3 comprising SEQ ID NO: 690;
[0568] b) a heavy chain variable region comprising SEQ ID NO:652 and a light chain variable region comprising SEQ ID NO:661;
[0569] c) a heavy chain comprising SEQ ID NO:640 and a light chain comprising SEQ ID NO:676;
[0570] d) a heavy chain comprising: HCDR1 comprising SEQ ID NO:662, HCDR2 comprising SEQ ID NO:663 and HCDR3 comprising SEQ ID NO:664, and a light chain comprising: comprising SE...
Embodiment
[0652] 1 De novo production and functional characterization of anti-CD3 mAbs
[0653] 1-1 Immunization with CD3 antigen to generate CD3 monoclonal antibodies
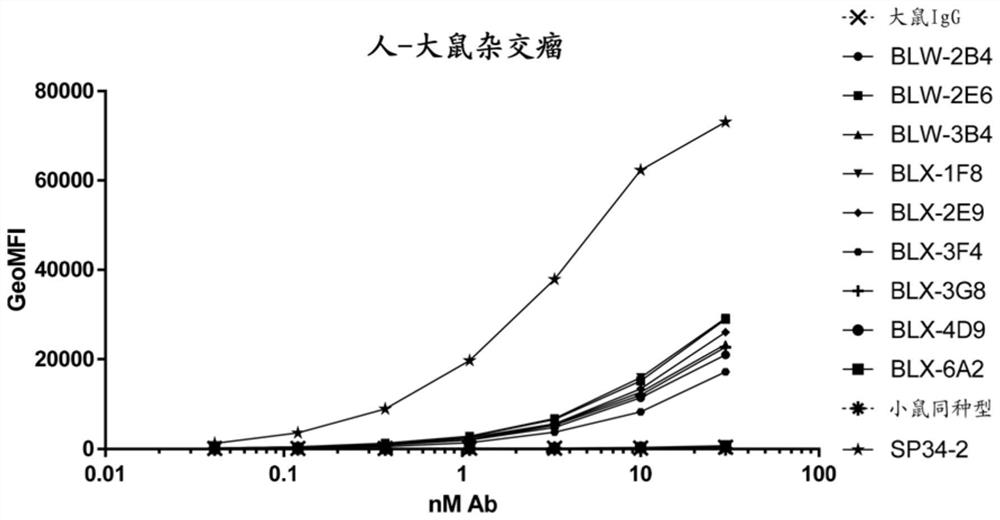
[0654] Immunization with a proprietary vector (Aldevron, Fargo, North Dakota, USA) encoding Human CD3e and Human CD3d; Cynomolgus CD3e and Cynomolgus CD3d. Animals received alternating boosts of human and cynomolgus DNA. From the 6th administration, animals received the optimized vector with the same insert. Cells from lymph nodes were fused with the Ag8 myeloma cell line. After IgM depletion, 40 million cells from confluent BLWs were plated on three 96-well plates. In the absence of IgM depletion, 133 million cells from confluent BLX were plated on nine 96-well plates.
[0655] Analysis of fusion BLW (bead depletion of lymphocytes) and BLX (bead depletion of lymphocytes) by cell-based ELISA (CELISA) on cells transiently transfected with human and cynomolgus cDNA cloned into the selection vector depleted)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com