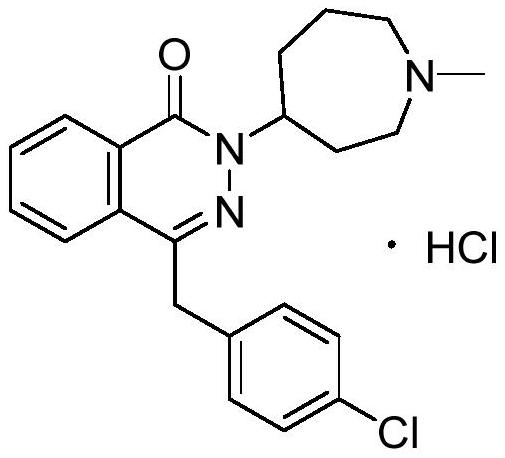
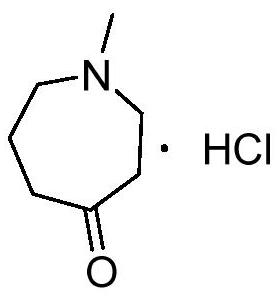
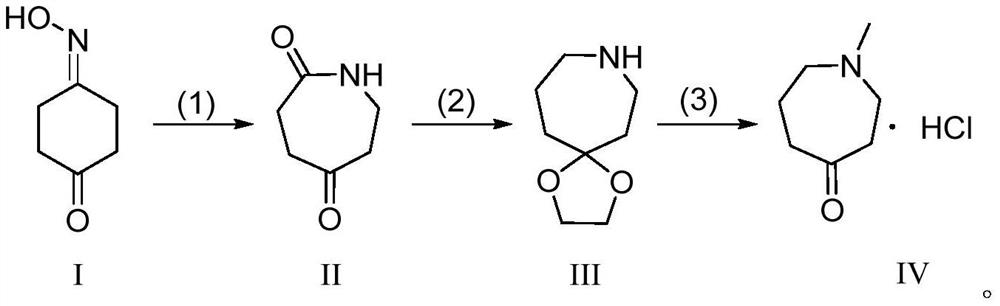
A method for preparing n-methylazepine-4-one hydrochloride
A technology of methylazepam and hydrochloride, which is applied in the field of medicinal chemistry, can solve the problems of limited industrial use, difficult storage, and high price, and achieve the effects of mild reaction conditions, cheap raw materials, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: synthetic reaction route is as follows:
[0042]
[0043] (1) Dissolve 4-oxime cyclohexanone (38.1g, 0.300mol) in 200mL of trifluoroacetic acid, trifluoroacetic acid is not only used as a catalyst to catalyze the Beckmann rearrangement, but also as a solvent, and the reaction solution is heated to 77°C, After refluxing for 1 hour, the trifluoroacetic acid was spin-dried and recrystallized from ethyl acetate to obtain 35.5 g of azepane-2,5-dione with a yield of 93%.
[0044](2) Add azepane-2,5-dione (31.8g, 0.250mol) to toluene 200mL, p-toluenesulfonic acid (2.2g, 0.013mol), ethylene glycol (23.3g, 0.375mol), Heat to 120°C, reflux for 2 hours to remove water, wash the solution successively with saturated sodium carbonate aqueous solution and saturated brine, dry and filter with anhydrous sodium sulfate, add red aluminum (416.6mL, 3.6mol / L toluene solution, 1.500mol), after the reaction solution was stirred at room temperature for 5 hours, 250mL of 5N aq...
Embodiment 2
[0046] Embodiment 2: synthetic reaction route is as follows:
[0047]
[0048] As described in Example 1, the difference is that the reagent used for Beckmann rearrangement in step (1) is p-toluenesulfonyl chloride, and the solvent is acetone. Dissolve 4-oxime cyclohexanone (38.1g, 0.300mol) in 500mL of acetone, add 180mL of 4N sodium hydroxide solution, then slowly add p-toluenesulfonyl chloride solution (102.9g, 0.540mol, dissolved in 500mL acetone), the reaction solution was stirred at room temperature for 3 hours, the acetone was evaporated, extracted with dichloromethane 3×200mL, dried over anhydrous sodium sulfate, filtered and spin-dried, and recrystallized with ethyl acetate to obtain 36.6g nitrogen Heteracycloheptane-2,5-dione, the yield is 96%.
Embodiment 3
[0049] Embodiment 3: synthetic reaction route is as follows:
[0050]
[0051] As described in Example 1, the difference is that the reagent used for Beckmann rearrangement in step (1) is concentrated sulfuric acid, and 4-oxime cyclohexanone (38.1 g, 0.300 mol) is slowly added to 100 mL under ice bath conditions Concentrated sulfuric acid, after adding, add this mixture into 100mL concentrated sulfuric acid which has been preheated to 120°C, react for 10 minutes, cool it with an ice bath, then add it dropwise into concentrated ammonia water, and use concentrated ammonia water After adjusting the pH to 6, extract with dichloromethane 3×200mL, dry and filter with anhydrous sodium sulfate, spin dry, and recrystallize from ethyl acetate to obtain 32.4 azepane-2,5-dione with a yield of 85% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com