Non-immunogenic engineered tissue and methods of producing and using the same
A non-immunogenic, engineered technology, applied in biochemical equipment and methods, tissue culture, bone/connective tissue cells, etc., can solve problems such as transplant rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
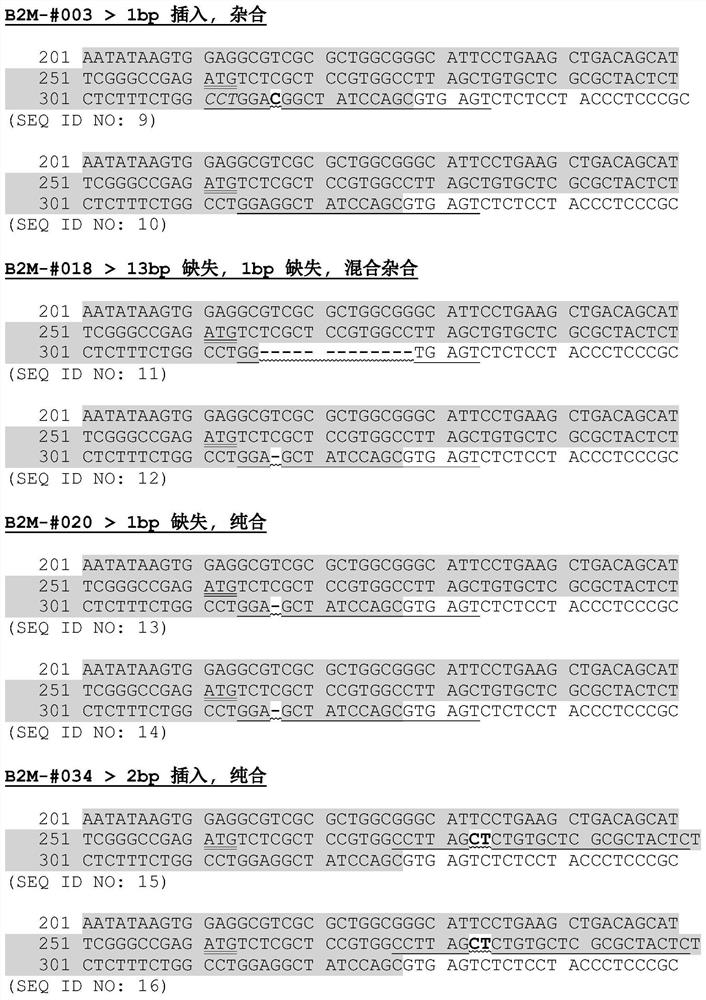
[0092] Example 1: Production of MHC I molecules and more stem cells containing immunomodulatory proteins in their surface
[0093] β2-microglobulin (B2M) knockout
[0094] A multi-capable cell line 50039 is obtained from NINDS human cells and databases. The cell line can also be obtained from Lonza, Baggbaderane et al. (Baggbaderane et al. (2015), STEM CELL Reprots, 5: 647-6659). Baggbaderane et al. Also disclose standard conditions for maintaining the cell line. Unlike this, the pluripotent stem cells are in STEMMACS TM Maintenance in the IPS-BREW medium. The extracellular matrix is provided using CTG layer adhesion protein-521 (Biolamina) or GelTrex (Thermo Scientific).
[0095] In order to destroy all the copies of the B2M gene, in accordance with the manufacturer's instructions, Integrated Dnatechnologies is used. CRISPER / CAS technology. Depending on the sequence of Crispr-Cas9 crrna, Alt-R Cas9 nuclease can specifically introduce double-strand breakage, which may result...
Embodiment 2
[0111] Example 2: Producing biological engineering myocardial tissue with modified PSC
[0112]The scheme described in WO 2015 / 040142 and Tiburcy et al. (Tiburcy, (2017), Circulation, 135: 1832-1847), and WO 2015 / 025030, starting from PSC, engineered myocardium begins. The plurality of stem cells, particularly clones, 18, 20, and 34, in Example 1, can be used in this embodiment. The scheme includes the step of induced induced embryo layers, cardiac differentiation, and heart maturation as described in WO 2015 / 040142, which is then directed to form a tissue in type I collagen hydrogel as described in WO 2015 / 025030.
[0113] In the first step, the PSC must differentiate into cardiomyocytes. This step can be carried out in accordance with, for example, Tiburcy et al. (Tiburcy et al., (2017), Circulation, 135: 1832-1847, and the first method disclosed in WO 2015 / 040142. Embodiment 1 Multi-capable cells (PSC) can be 5 × 10 on the masstel of 1:30 4 To 1 × 10 5 Cell / cm 2 Plate on a p...
Embodiment 3
[0119] Example 3: A modified PSC produces biological engineered myocardial tissue
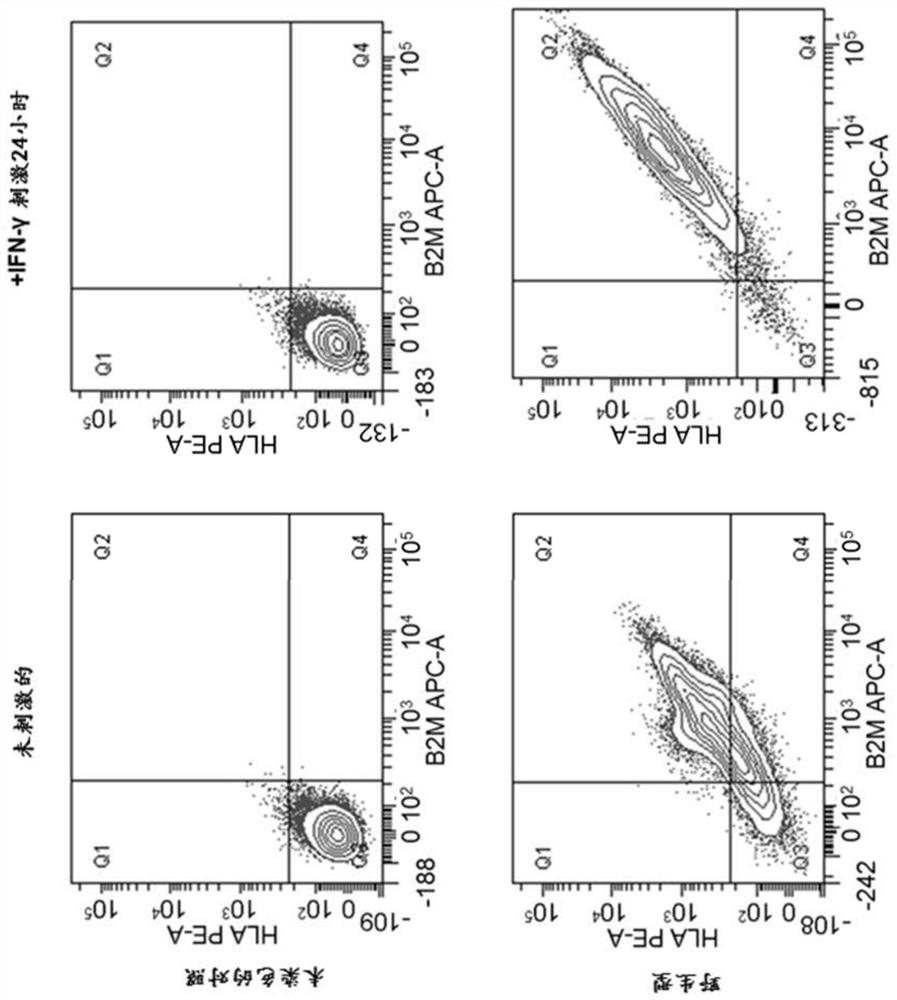
[0120] The HIPSCs (see Example 1) obtained herein are differentiated as cardiomyocytes (CM) based on the protocols described in Example 2 described above (Tiburcy et al., 2017, WO 2015 / 025030). HLA-E ki HIPSC derived CMS showed the expression of muscle subinoproteins; α-ciliacin and myocardial kerceculin T (CTNT), together with> 90% cuisurine protein + cell high purity ( Figure 10 A).
[0121] Flow cytometry analysis showed wild type (WT) CMS expression B2M and HLA I molecules; HLA-B and C, but did not express HLA-E under basic conditions. IFN-γ treatment induces B2M and HLA-B, C molecules, and HLA-E is slightly lower (~ 60%). As a negative control, if expected, B2M KO CMS displays no HLA expression. HLA-E ki cloned (dimer and trimer) expressed B2M and HLA-E after IFN-γ, and did not show any other HLA I expression. CMS from HLA-E trimer HIPSC is slightly high in initial analysis compared to the co...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap