Nucleic acid antibody kit for rapid detection of virus
A kit and antibody technology, applied in the field of virus diagnosis, can solve the problems of unfavorable patient treatment, affecting the detection rate, inability to detect hepatitis B virus early and accurately, and achieve the effect of facilitating treatment and improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Preparation of anti-HBsAg antibody
[0037] Take the HBV surface antigen protein, dilute the antigen to 1mg / ml, take 50μl of antigen solution, 50μl of 0.01M PBS and 150μl of Freund's complete adjuvant for the first immunization, mix and emulsify it, and subcutaneously immunize male BALB / c children aged 6-8 weeks at multiple points mouse. After the initial immunization, every two weeks, take 25 μl of antigen solution, 75 μl of 0.01M PBS and 100 μl of Freund's incomplete adjuvant and mix them for 5 booster immunizations. The spleen was taken four days after the last immunization for cell fusion. The splenocytes of the immunized Balb / c mice were taken and fused with the myeloma Sp2 / 0 cell line using the PEG method. The fused cells were resuspended in 20% FBS-HAT-DMEM medium, and evenly spread on 96 Inside the orifice plate, 37°C, 5% CO 2 nourish. The fused cells were cultured for about a week and replaced with 10% FBS-HT-DMEM medium in half. When the area of...
Embodiment 2
[0038] Example 2. Purification of monoclonal antibodies
[0039] BALB / c mice were taken, and 1 week before hybridoma cell inoculation, pristane was injected intraperitoneally, 0.5ml / mouse. After 1 week, each mouse was inoculated intraperitoneally with about 1X10 6 hybridoma cells; 7-10 days later, ascites was collected. Centrifuge the ascitic fluid at 10,000×g for 30 minutes, discard the precipitate, use 50% ammonium sulfate for salting out and extract roughly, dissolve it in PBS, and dialyze with running water for 5 hours; dialyze with 0.1mol / L phosphate buffer (pH8.0) overnight; load the sample , use 0.1mol / L phosphate buffer (pH8.0) to elute the impurity protein, elute with citrate eluent with different pH values, collect the elution peaks in sections, and concentrate to obtain the purified anti-HBsAg Antibody 5G7-2.
Embodiment 3
[0040] Example 3. Anti-HBsAg antibody subtype identification
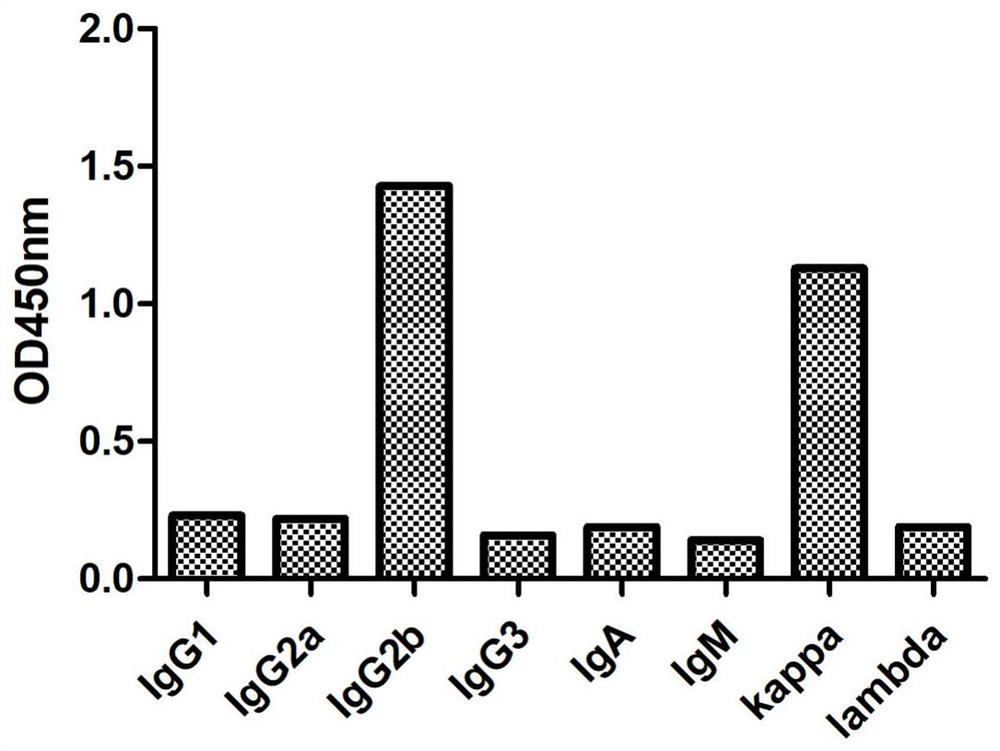
[0041] Subtype determination was performed on the positive mouse monoclonal cell lines screened by indirect ELISA according to the subtype determination reagent (Sigma Company). The ELISA plate provided in the kit has been pre-coated with specific antibodies against mouse IgG1, IgG2a, IgG2b, IgG3, IgA, IgM, kappa light chain, lambda light chain, and the anti-HBsAg purified in Example 2 Antibody 5G7-2 samples were added to the sample wells, 50 μl per well, without incubation. Add 1X goat anti-mouse IgA+IgM+IgG-HRP into the sample wells, 50 μl per well, mix gently, and incubate for 1 h. Remove the liquid in the well and add 1XPBST to wash the well 3 times, and absorb excess water with absorbent paper. Add chromogenic solution, 100 μl per well, and develop color for 15 minutes at room temperature in the dark. Add 100 μl stop solution to stop the color reaction. The result is as figure 1 As shown, the monoclonal a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com