Stable aqueous composition comprising oligosaccharides
A composition and oligosaccharide technology, which can be applied in the directions of drug combination, medical preparations containing active ingredients, drug delivery, etc., and can solve problems such as being unsuitable for administration to infants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0051] Oligosaccharides
[0052] In one embodiment, the aqueous composition according to the invention comprises two or more oligosaccharides having a glucose unit at the reducing end.
[0053] In one embodiment, the aqueous composition according to the invention comprises two oligosaccharides having a glucose unit at the reducing end.
[0054] In one embodiment, the aqueous composition according to the invention comprises three oligosaccharides having a glucose unit at the reducing end.
[0055] In one embodiment, the aqueous composition according to the invention comprises five oligosaccharides having a glucose unit at the reducing end.
[0056] In one embodiment, the aqueous composition according to the invention comprises six oligosaccharides having a glucose unit at the reducing end.
[0057] In one embodiment, the aqueous composition according to the invention comprises seven oligosaccharides having a glucose unit at the reducing end.
[0058] In one embodiment, in ...
Embodiment 1
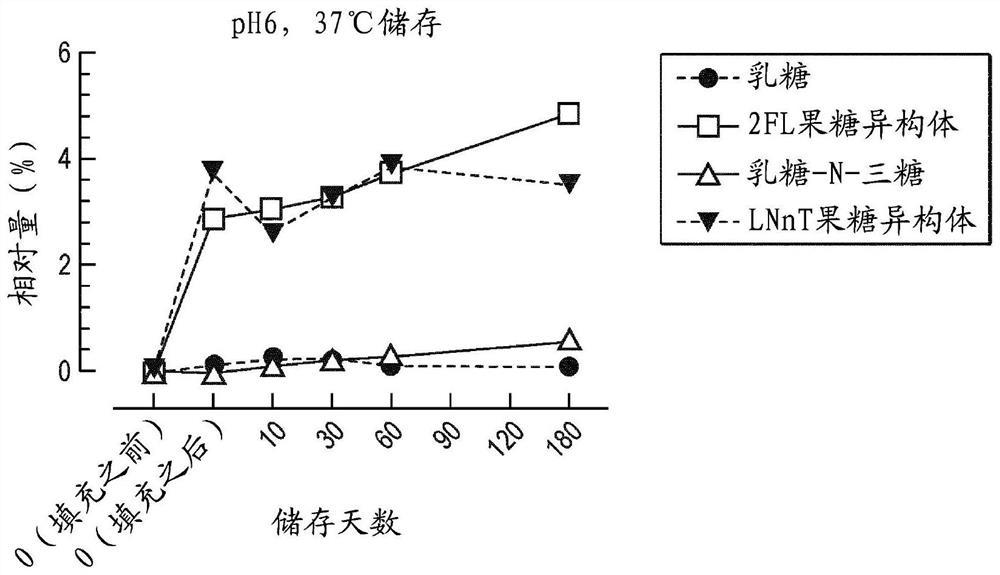
[0164] The oligosaccharides 2'fucosyllactose (2'FL) and lactose-N-neotetraose (LNnT) were mixed in a 2:1 ratio (w / w) and dissolved in water to 10% (w / v) final concentration. The solution was acidified to pH 6 with citric acid and subjected to heat treatment (UHT) before aseptically filling small single-dose vials. The solutions were analyzed before and after filling the vials and after storage at 37°C for various times (accelerated storage conditions).
[0165] Analysis was performed by HPLC using a TSK Gel Amide-80 (150 mm×4.6 mm, particle size: 3 μm) column and detected via a charged aerosol detector. The relative response of the oligosaccharides to the standard curve was quantified using the added fructose isomers of 2'FL and LNnT and their degradation products (fucose, lactose and lacto-N-triose). Fucose, lactose, 2FL, LNnT, 2'-fucosyl-lactulose and LNnT fructose isomers were used as standards. The lactose-N-triose content was calculated using the calibration curve of L...
Embodiment 2
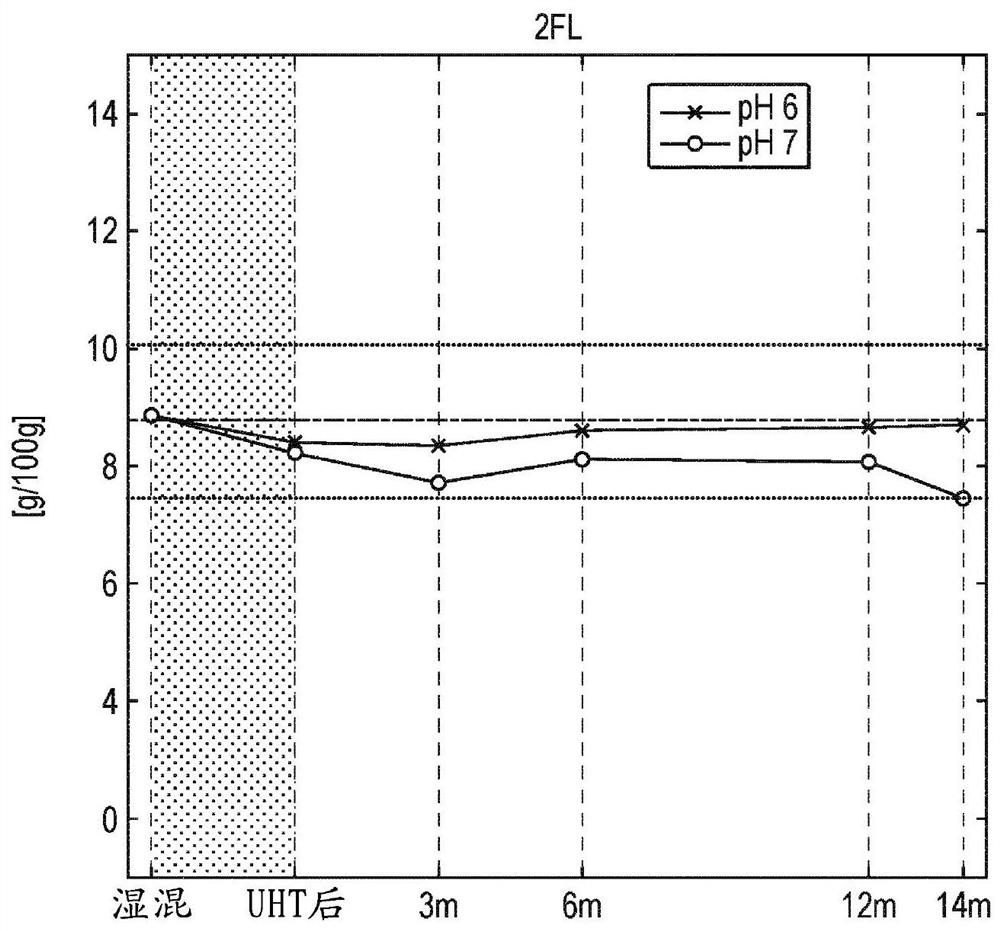
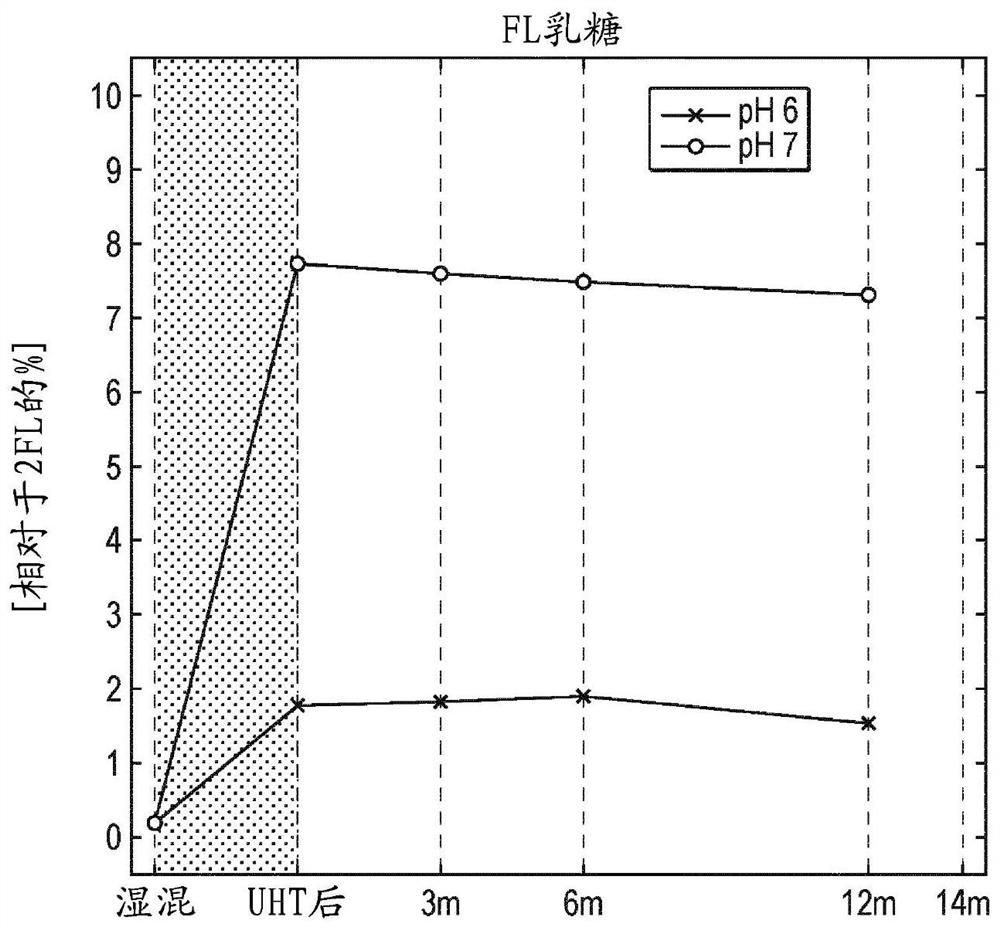
[0168] The oligosaccharides 2'fucosyllactose (2'FL) and lactose-N-neotetraose (LNnT) were mixed in a 10:1 ratio (w / w) and dissolved in water to 10% (w / v) final concentration. From this solution, three samples were generated with pH 6 and pH 7 respectively (by addition of KOH). Such samples were subjected to heat treatment (UHT) before aseptically filling small single-dose vials. The solutions at different pH were analyzed before and after filling the vials and after storage at room temperature (21-26° C.) for various times. Analysis was performed by HPLC using a TSK Gel Amide-80 (150 mm×4.6 mm, particle size: 3 μm) column and detected via a charged aerosol detector. F or 2'FL (Fig. 2a), LNnT (Fig. 2d), the added fructose isomer of 2'FL (indicated as FL lactulose in the figure, Fig. 2d) and the added fructose isomer of LNnT (in The LNnT isomers, Fig. 2e) and their degradation products (lactose, Fig. 2c) are indicated in the figure. The pH of the solution was also measured. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com