Anti-c-met antibody showing enhanced stability or antigen-binding fragments thereof
A technology of hepatocyte growth factor and binding fragment, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, specific peptide, drug combination, etc., can solve problems such as structural instability, and achieve production cost reduction , the effect of reducing immunogenicity and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0167] Throughout this specification, unless otherwise mentioned, "%" used to express the concentration of a specific substance is (weight / weight)% in solid / solid, (weight / volume) in solid / liquid % and (v / v)% in liquid / liquid.
[0168] Example 1. Constant region substitution and biological activity confirmation of 1E4 antibody
[0169] In order to maintain the characteristics of the anti-c-hepatocyte growth factor receptor antibody in 1E4, which is an IgG1-based anti-c-hepatocyte growth factor receptor antibody disclosed in conventional patents WO2016 / 021864A1 and WO2017 / 135791A1 To improve the structural stability, the present inventors utilized a complementarity-determining region grafting method in which the complementarity-determining region of a conventional antibody is grafted to a framework region that is more stable than that of a conventional antibody. Before implementing the complementarity-determining region transplantation method, ABZENA Company of the United King...
Embodiment 2
[0172] Example 2. Searching for the optimal framework (framework) for CDR transplantation
[0173] In relation to 1E4, which is an IgG1-based anti-c-hepatocyte growth factor receptor antibody disclosed in previous patents WO2016 / 021864A1 and WO2017 / 135791A1, the present inventors intend to maintain the anti-c-hepatocyte growth factor receptor antibody characteristics while improving structural stability. For this reason, the present inventors utilized a complementarity-determining region grafting method in which the complementarity-determining region of a conventional antibody is grafted to a framework region that is more stable than that of a conventional antibody.
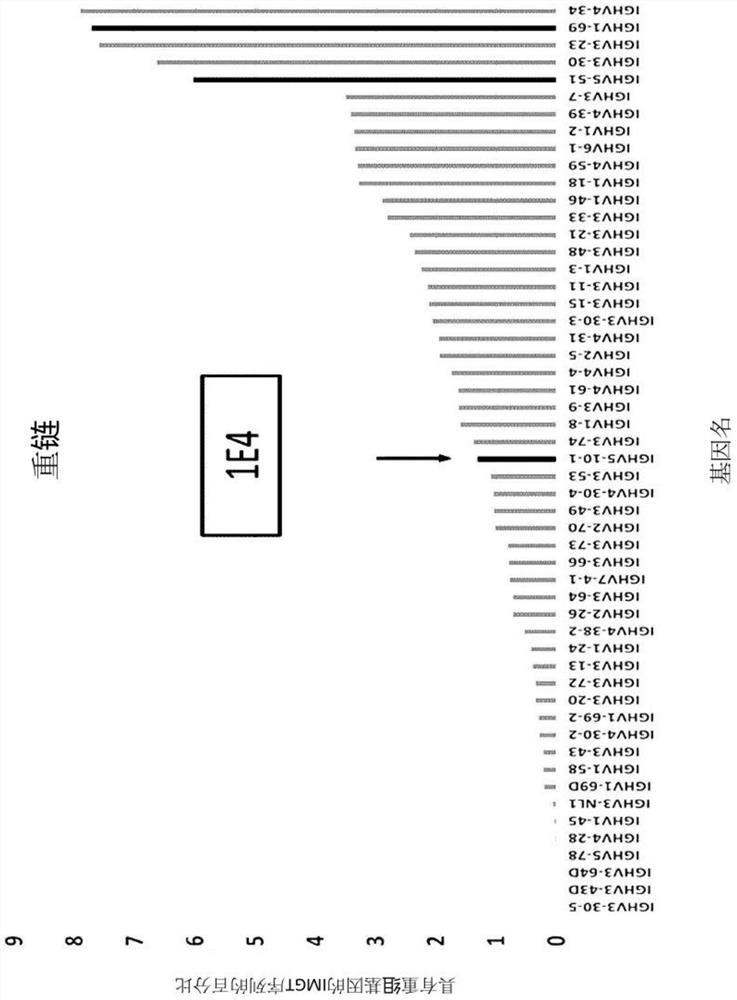
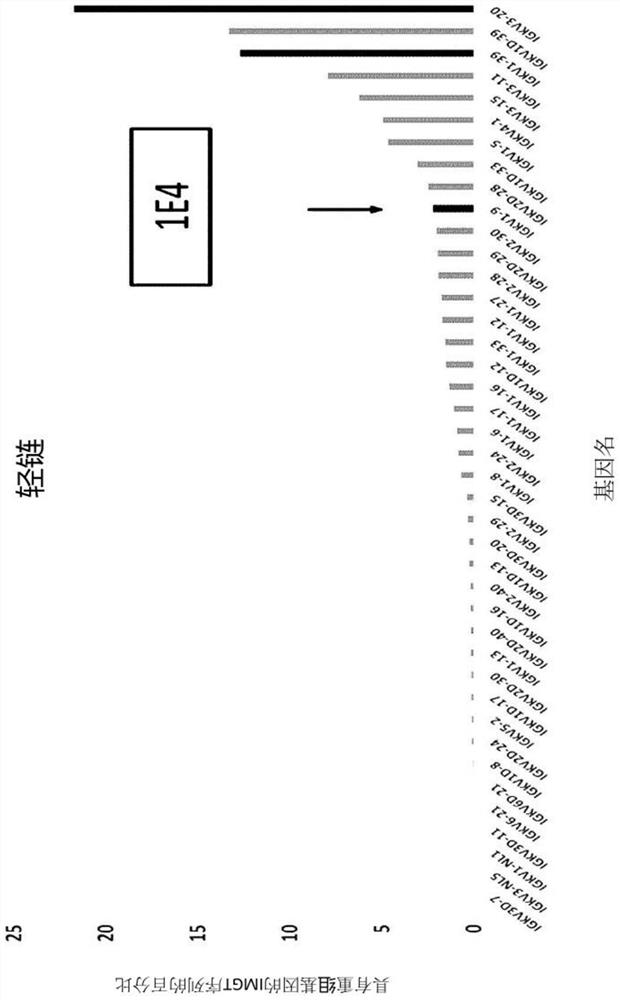
[0174]First, in order to screen the optimal framework region, the heavy chain variable domain (hereinafter referred to as "VH") and the light chain variable domain (hereinafter referred to as "Vk") The amino acid sequence of the site was compared with a database of human germline variable (V) and junction (J) fr...
Embodiment 3
[0202] Example 3. Establishment of final candidate heavy chain variable region and light chain variable region sequences evaluated by In silico immunogenicity
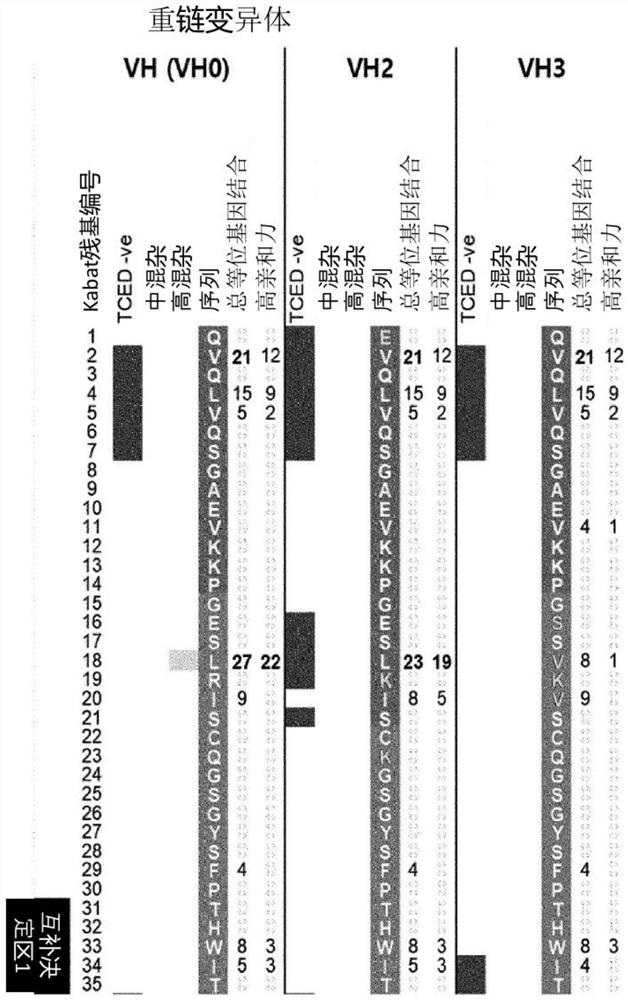
[0203] The complementarity determining region of the 1E4 antibody was grafted to the backbone screened in Example 2, and the amino acid residues confirmed to be important for maintaining the antigen-antibody binding force were back-mutated (back-mutated) using iTope from ABZENA, UK. TM and TCED TM (Perry et al. 2008; Bryson et al. 2010) Analytical techniques to evaluate in silico immunogenicity.
[0204] Specifically, among the peptides (peptides) of the backbone candidate sequences screened in Example 2, 9 peptides were added as one amino acid unit and scanning analysis was performed. According to this analysis technique, the amino acid residues of antibody variable region sequences are numbered using the Kabat numbering system, in "iTope TM The " column indicates regions containing potentially immunogenic peptides....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com