Application of Vpr protein N-terminal amino acid polypeptide for regulating and controlling tumor cell apoptosis in preparation of antitumor drugs
A tumor cell apoptosis, anti-tumor drug technology, applied in anti-tumor drugs, drug combinations, peptide/protein components, etc., can solve the problems of poor specificity of action, single means, and easy to produce drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
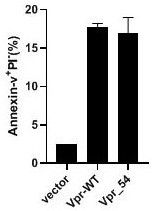
[0019] To study the activity and function of natural truncated VPR, wild-type VPR-WT and truncated Vpr_54 (54-bit amino acid termination) expression vector parallel to the TZM-BL cell line (cervical cancer cell line) were transfected parallel. like figure 1 As shown, the apoptosis of the TZM-BL cells of wild type (VPR-WT) or TZM-BL cells (ANNEXIN-V + / Pi-) increased (Annexin-V + / Pi-) by transfected wild-type (VPR-WT) or TZM-BL cells of VPM-BL (VPR_54) ( 15%, P 0.05). Tip truncation VPR also has the same projection activity.
Embodiment 2
[0021] In order to determine the specific location of the N-terminal apoptotic motif of the VPR protein N-terminal, a series of VPR orientation site mutations were constructed. These mutations are uncertain mutations in the position of VPR protein amino acids, 38, 34, 30, 27, and 23, subsequently transfected into TZM-BL cells. The truncated VPR containing a complete first spiral structure produced similar proximity activity compared to tumor cells transfected carrier-controlled tumor cells. These results show that there is a cell death region in the first spiral (23AA-37AA) of the VPR N-terminal.
Embodiment 3
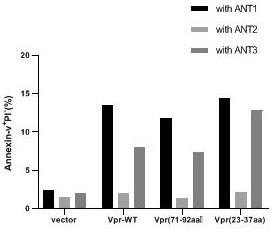
[0023] Wild VPR-WT and VPR (71-92 AA), VPR (71-92 AA) respectively act in tumor cells together with adenine nucleotide, Ant-3, Ant-2 to tumor cells. Experimental results figure 2 The experimental results show that the N-terminal cell death domain (23-37AA) and known C-terminal cell death domains (71-92AA) have the same ability to promote apoptosis, and this proceeds Ability is associated with different ANT subtypes, and Over-expression of Ant-1 and Ant-3 can promote VPR-mediated tumor cell apoptosis, while Ant-2 overexpression can inhibit the acort of Vpr.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com