Anti-human nkx3.1 monoclonal antibody and its preparation method and application
A monoclonal antibody and sequence technology, applied in the field of immunology, can solve the problems of specificity and stability to be improved, high price, and large differences between antibody batches, so as to improve specificity and affinity, improve immune hit rate, The effect of stabilizing antibody properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of immunogen
[0039] 1. NKX3.1 head and tail selection
[0040] The head and tail sequences were selected based on the NKX3.1 sequence and structure published by Uniprot for fusion expression of NKX3.1. The accession number of the Uniprot of human NKX3.1 is Q99801, which contains 234 amino acids, of which 1-123 is its head, 124-183 is its stem, and 184-234 is its tail. The amino acid and nucleic acid sequences of the head are as shown in SEQ respectively. ID NOs: 11 and 12, and the tail amino acid and nucleic acid sequences are shown in SEQ ID NOs: 13 and 14, respectively.
[0041] 2. Fusion expression and purification
[0042] 1) The head and tail nucleic acid sequences of NKX3.1 were artificially synthesized, and enzyme cleavage sites NdeI and XhoI were introduced at both ends of the sequence. After synthesis, cloned into the pET-28a expression vector to construct pET-28a-NKX3.1 -A, pET-28a-NKX3.1-B expression vector.
[0043] 2) The recombina...
Embodiment 2
[0047] Example 2 NKX3.1 hybridoma cell line screening and monoclonal antibody preparation
[0048] 1. Animal immunity:
[0049] The NKX3.1-A and NKX3.1-B proteins purified above were mixed with an equal volume of Freund's complete adjuvant respectively, and the immunization dose of 6-8 weeks old BalB / c mice was 100 μg by subcutaneous injection. Each animal was immunized for the second time after an interval of two weeks. The antigen was emulsified with incomplete Freund's adjuvant, and the immunization dose was the same as that of the first time. After the second immunization, the tail blood was collected and the serum titer was determined by indirect ELISA method.
[0050] 2. Cell fusion:
[0051] Myeloma cells use sp2 / 0 derived from BalB / c, which is in the logarithmic growth phase when fused; the spleen of mice after shock immunization is aseptically harvested to prepare a single-cell suspension of spleen cells; mouse spleen cells and myeloma cells Mix at a ratio of 1:5, ...
Embodiment 3
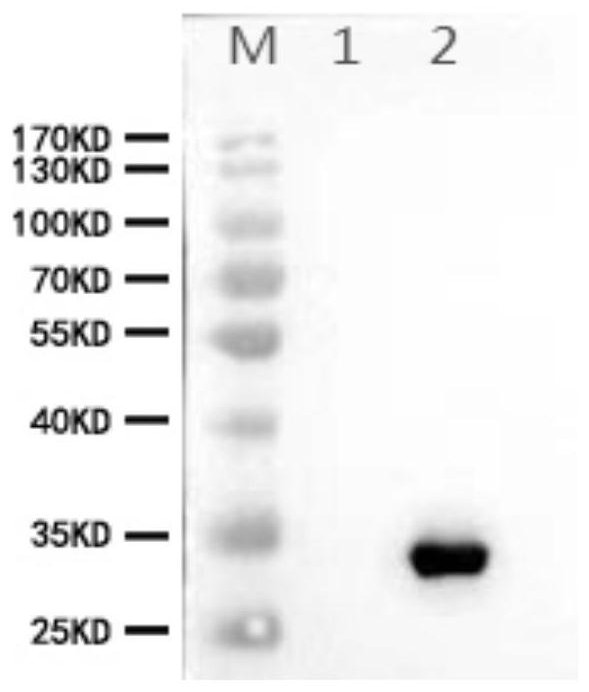
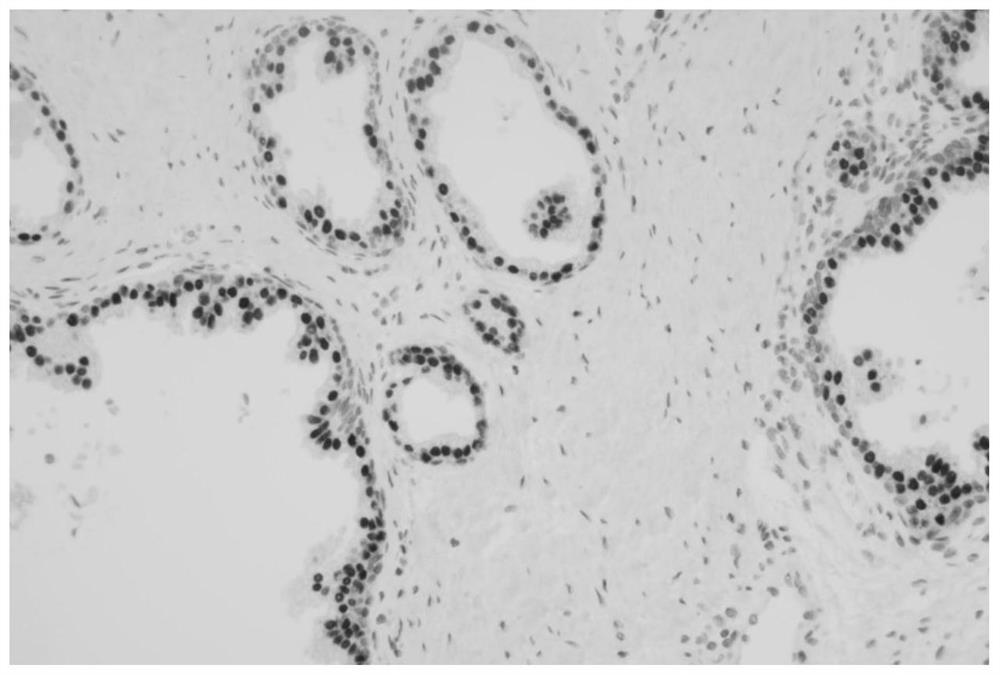
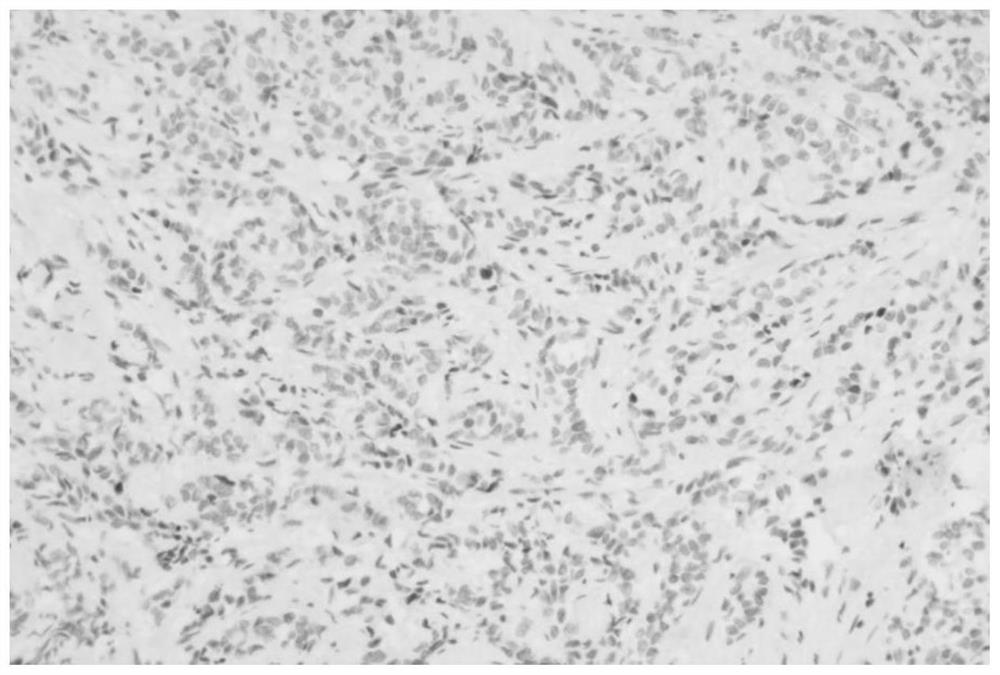
[0059] Example 3 Detection of specificity of NKX3.1 monoclonal antibody by Western-Blot
[0060] 1. Sample preparation: prepare sp2 / 0 cell lysate and LNCaP cell lysate, lyse with RAPI containing 1mM PMSF, and add 5XSDS-PAGE Loading buffer for sample preparation. Sp2 / 0 is a mouse myeloma cell without fusion as a negative control, LNCaP cells are a human prostate cancer cell as a positive control, the two lysates will release intracellular proteins, and mouse myeloma cells will not express NKX3.1 , and LNCaP cells are cells that specifically express NKX3.1, which will bind to the screened positive monoclonal antibodies, and WB will show a positive reaction.
[0061] 2. Electrophoresis and membrane transfer: Load 30μg of each sample, run at 80V for 20min, and then run electrophoresis at 120V to bromophenol blue to the bottom of the gel. Then start to transfer the membrane, soak the NC membrane and filter paper with transfer buffer in advance, and place the sandwich form on the w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com