Anti-human interleukin-4 receptor alpha antibody as well as preparation method and application thereof
A technology of interleukin and antibody, applied in botany equipment and methods, biochemical equipment and methods, anti-animal/human immunoglobulin, etc., can solve problems such as immune system disorders, side effects, and patients with no cure , to achieve strong asthma suppression function and rapid onset effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0091] In some embodiments, the preparation method of the anti-human IL-4Rα monoclonal antibody disclosed herein comprises: cultivating host cells under expression conditions, thereby expressing the anti-human IL-4Rα monoclonal antibody; isolating and purifying the expressed anti-human IL-4Rα -4Rα monoclonal antibody. Using the above method, the recombinant protein can be purified into a substantially uniform substance, such as a single band on SDS-PAGE electrophoresis.
[0092] In some embodiments, the anti-IL-4Rα antibody disclosed herein can be separated and purified by affinity chromatography. According to the characteristics of the affinity column used, conventional methods such as high-salt buffer, changing pH and other methods to elute the anti-IL-4Rα antibody bound to the affinity column.
[0093] In some embodiments, the humanized anti-human IL-4Rα monoclonal antibody disclosed herein is obtained by immunizing Ba1b / c mice with IL-4Rα antigen prepared in the laborator...
Embodiment 1
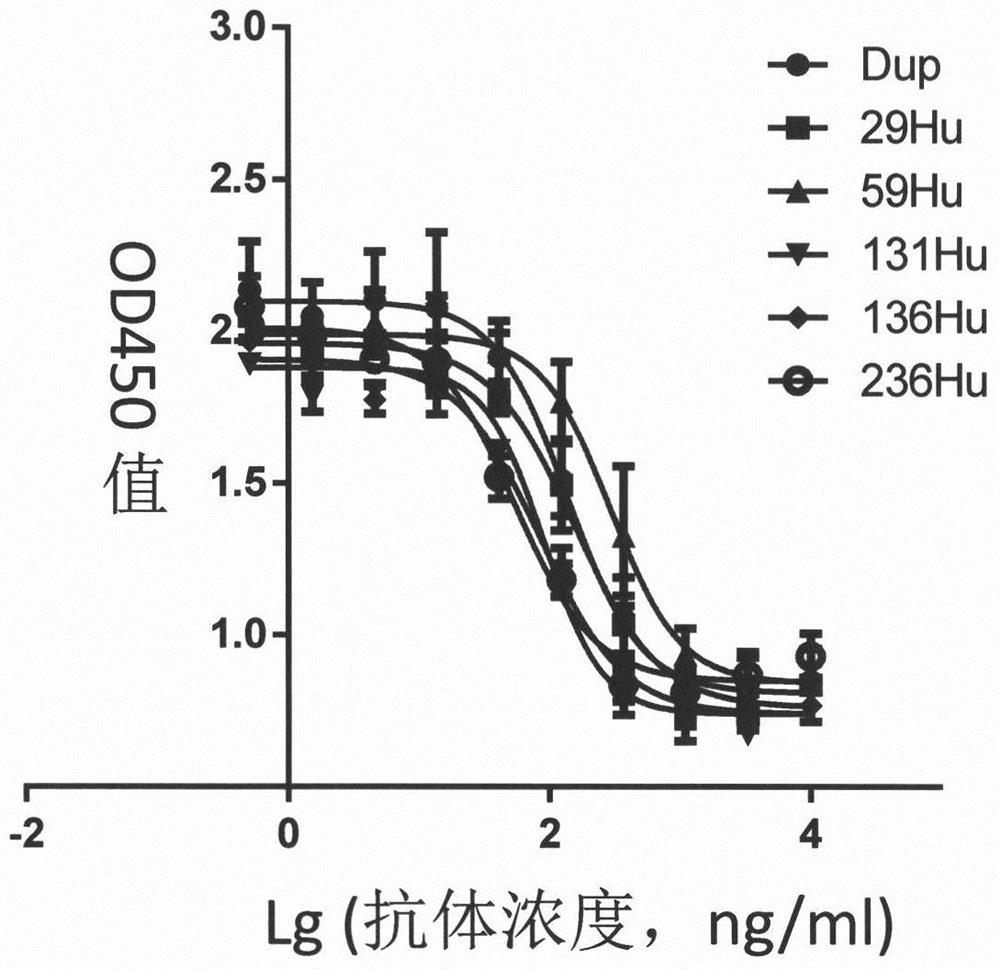
[0108] Example 1 Soluble IL-4Rα extracellular segment Fc tag and Flag tag antigen, reference antibody Dupilumab and IL-4 preparation of
[0109]The human IL-4Rα extracellular segment antigen sequence was obtained from UniProt (UniProtKB-P24394). Codon optimization was performed according to the codon usage preference of Cricetulus griseus, and the gene synthesis of the 26-232 amino acid fragment at the N-terminal was carried out, and the sequence was subcloned into pUC57 In the vector, pUC57-hIL4Rα-ECD was obtained. The constant region sequence of human IgG1 was synthesized according to the sequence of Secukinumab (see WHO Drug Information Vol. 23, No. 4, 2009. P342). The sequence was subcloned into pUC57 vector to obtain pUC57-IgG1-CH. The hFc fragment and the Flag tag (DYKDDDDK) were inserted into the C-terminal of the hIL4Rα-ECD fragment by PCR method, and constructed on the pTT5 expression vector (preserved in the laboratory) to obtain pTT5 (hIL4Rα-ECD-hFc) and pTT5 (...
Embodiment 2
[0113] Example 2 Immunization of hIL-4Rα-ECD-Fc
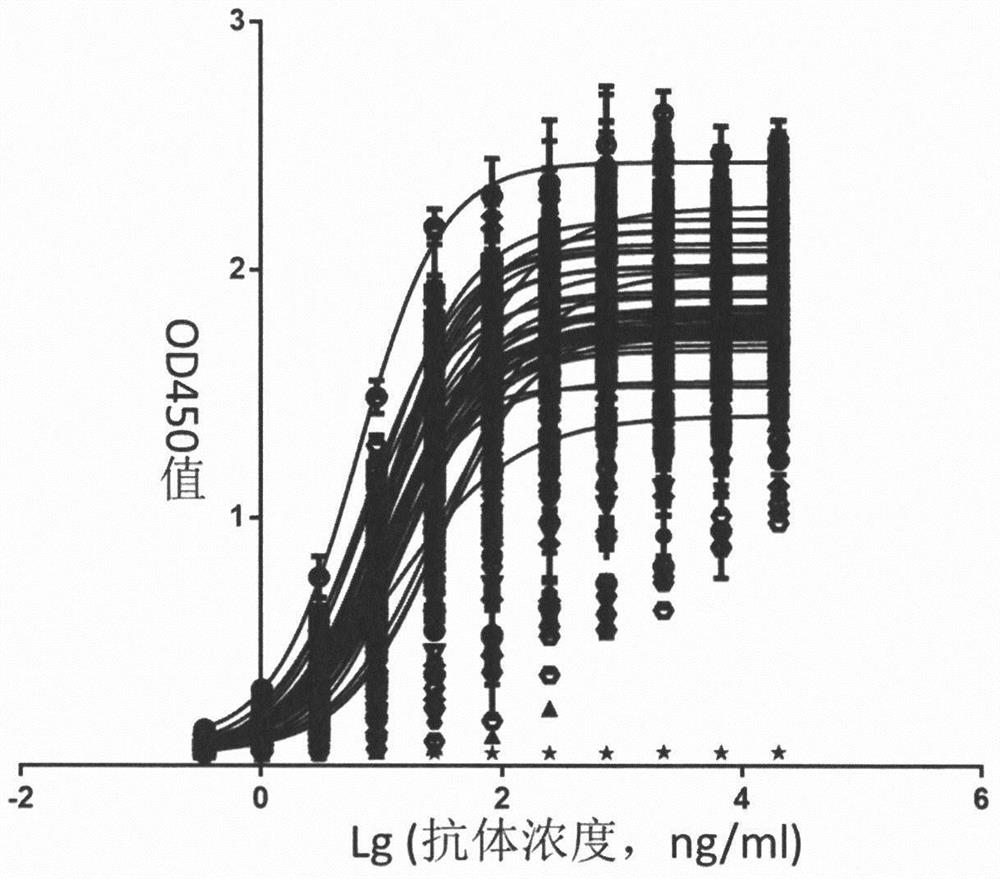
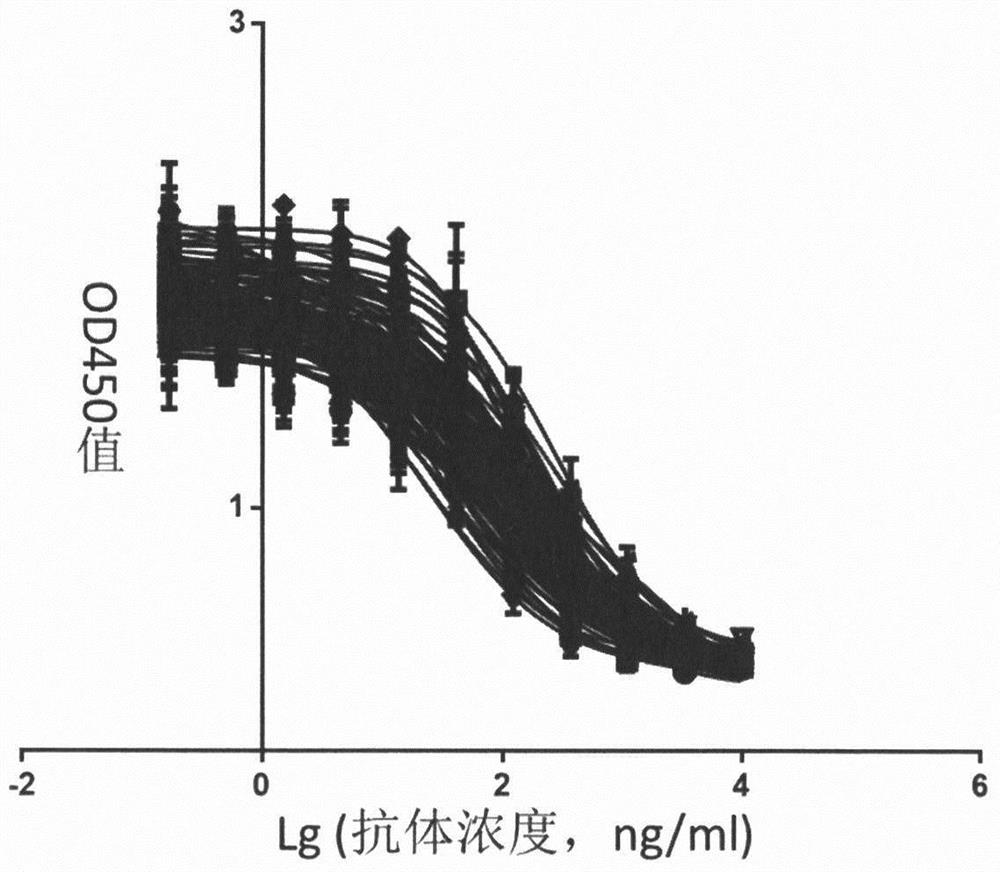
[0114] Dilute 100 μg / mouse hIL-4Rα-ECD-Fc antigen with physiological saline to 75 μl, mix with an equal volume of Freund’s complete adjuvant, and complete phacoemulsification for 4-5 week-old Balb / c mice (purchased from Shanghai Lingchang Biotechnology Co., Ltd., animal production license number: SCXK (Shanghai) 2013-0018) for subcutaneous multi-point injection. Three weeks later, 50 μg / mouse protein was also diluted to 75 μl and mixed with an equal volume of Freund’s incomplete adjuvant. After phacoemulsification was complete, the mice were injected subcutaneously at multiple points, and the immunization was repeated two weeks later. One week after the third immunization, the tails of all mice were cut to collect blood to separate serum, and the serum titer was detected by ELISA coated with hIL-4Rα-Fc antigen. For mice with serum antibody titer > 10000, impulse immunization was performed one week after blood collection: 10 ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap