Methods for labeling eukaryotic cells from multicellular organism as well as for treating and/or diagnosing cancer using modified monosaccharide compounds
A technology of eukaryotic cells and compounds, applied in the medical field, can solve problems such as assimilation of monosaccharide compounds that cannot be explained
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
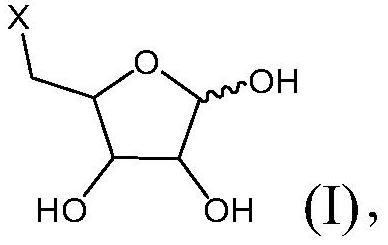
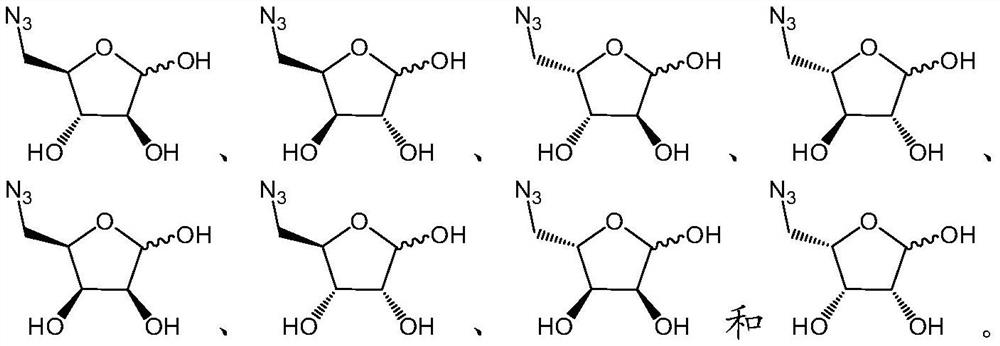
[0151] Example 1 : Synthesis of Compounds
[0152] Materials and methods:
[0153] Thin layer chromatography was performed on a Merck 60F254 by UV, and / or by using sulfuric acid or KMnO 4 Or phosphomolybdic acid solution for carbonization to detect. Silica gel 60 40-63Mm is used for flash column chromatography.
[0154] NMR spectra were acquired on a Bruker Avance 300 or 500 MHz spectrometer using residual protonated solvent as internal standard. Chemical shifts δ are given in parts per million (ppm) and coupling constants are reported in Hertz (Hz). Splitting patterns are designated as singlet (s), doublet (d), triplet (t), doublet of doublet (dd), doublet of doublet of doublet (ddd). Splitting patterns that could not be explained or easily visualized were designated as multiplets (m).
[0155] Mass spectra were collected on a Waters LCT Premier XE (ToF) with electrospray ionization in positive (ESI+) or negative (ESI-) detection mode.
[0156] IR-FT spectra were reco...
Embodiment 2
[0223] Example 2 : labeling of bladder non-neoplastic and neoplastic eukaryotic cells
[0224] Materials and Methods:
[0225] Cell culture:
[0226] Human urothelial cells (SV-HUC-1) and human bladder carcinoma (T24) were purchased from ATCC (Manassas, Van USA). These cell lines were grown in RPMI 1640 with glutamine 1 medium (Lonza Biowhttaker) supplemented with 10% fetal bovine serum (FBS) (VWR International S.A.S). Medium was changed every two days. Cell passage was performed using Tryp-LE express1x (Gibco). Cell viability was estimated using the trypan blue exclusion assay.
[0227] Cells exposed to Ara-N 3 Probe:
[0228] SV-HUC-1 and T24 cells were seeded in 24-well plates at a density of 5*10 4 cells / well. Then, cells were incubated at 37°C, 5% CO 2 Incubate for 24 hours. Thereafter, the medium in each well was discarded, and the adherent cells were washed twice with phosphate-buffered saline (PBS) (Lonza Biowhittaker), and then exposed to Ara-N 3 Prob...
Embodiment 3
[0242] Example 3 : labeling of noncancerous and cancerous eukaryotic cells
[0243] Materials and Methods:
[0244] The same experiments as in Example 2 were performed on other cancers and corresponding non-cancerous cells. The medium was adapted to the tested cells and supplemented with 10% fetal bovine serum (FBS). Ara-N 3 Probe (10 mM) was added to the medium at FBS concentration (10%). The incubation time corresponds to one or two doubling times of the test cells (see table below).
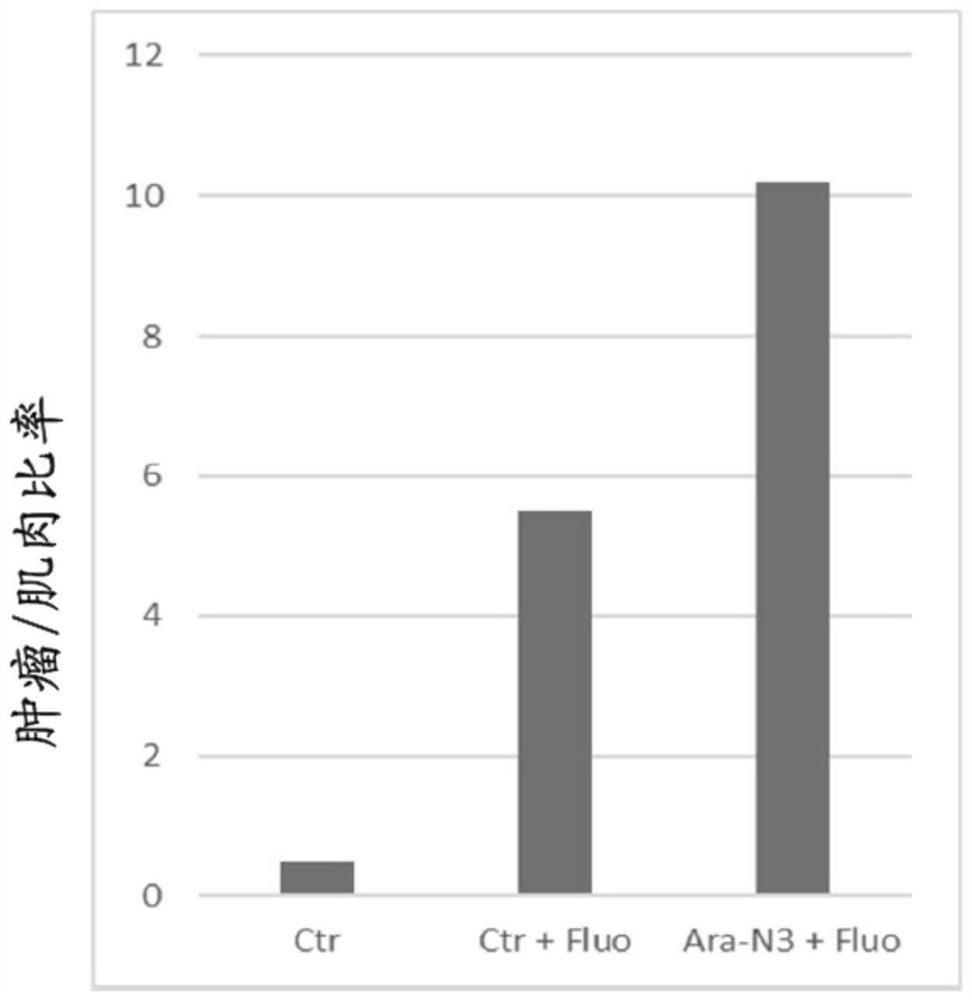
[0245] The fluorescent signal cancer / non-cancer ratio is based on the Ara-N 3 Calculated from fluorescence intensities obtained by testing on cancer and corresponding non-cancer cells.
[0246] result:
[0247] The results are shown below (Table 3), the cancer cell lines tested showed higher fluorescence intensity (ratio higher than 1) compared to the corresponding non-cancer cell lines.
[0248]
[0249]
[0250]
[0251] The same experiment was performed on other human canc...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap