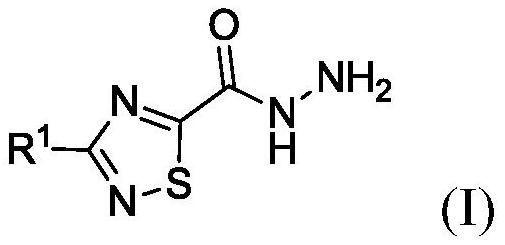
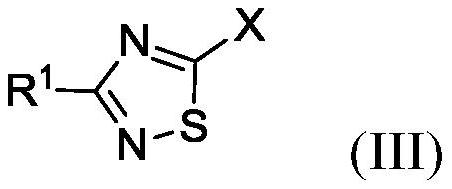
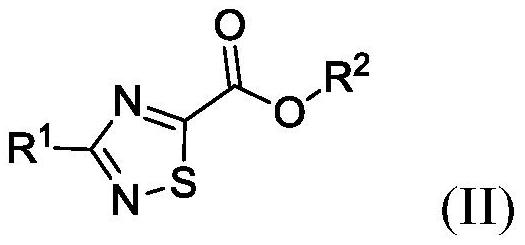
Synthesis of 3-methyl-1,2,4-thiadiazole-5-carbohydrazide and methyl-d3 deuterated form thereof
A methyl, V-2 technology, applied in the field of synthesis of compounds or their salts, can solve problems such as low overall yield of intermediates, adverse effects on drug product quality, raw material problems and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0336] Example 1A: Synthesis of deuterated d3-AMTD (IV-2) via the acetamidine route
[0337]
[0338] Pinner reaction via Pinner salt (VI-2) affords d3-acetamidine (V-2), followed by cyclization via the "bromine route" (step c1) or the "hypochlorite route" (step c2) to form compounds The thiadiazole ring of (IV-2).
[0339] Step a: Formation of Pinner salt (VI-2)
[0340] At 20-25°C, in a 750 mL three-necked glass flask equipped with two gas inlets, a thermometer and a magnetic stir bar, charged 74 mL of anhydrous diol and 51.2 g (1.161 mol, 1.0 eq) of d3-acetonitrile (VII -2) followed by cooling to 0-5°C (cooling by ice-salt mixture). Keeping the temperature below 10 °C, HCl gas was bubbled through the mixture for 6 hours. The reaction mixture was heated to 20-25°C and stirred for 16.5 hours (the mixture became a thick white suspension which was difficult to stir). 500 mL of TBME was added to the mixture, after which the mixture became an easily stirrable white suspe...
Embodiment 1B
[0348] Example 1B: Synthesis of deuterated d3-AMTD (IV-2) via the hydroxyacetamidine route
[0349]
[0350] d3-Hydroxyacetamidine (IX-2) is obtained from deuterated acetonitrile (VII-2). Afterwards, the corresponding tosyl intermediate (X-2) is cyclized in the presence of thiocyanate to form the thiadiazole ring of compound (IV-2).
[0351] Step a: Formation of d3-hydroxyacetamidine (IX-2)
[0352] Charge 5.5 mL (4.65 g, 105.4 mmol, 1.0 eq) of d3-acetonitrile (d: 0.844 g / mL) at 20-25 °C in a 100 mL glass container equipped with a reflux condenser, thermometer and magnetic stir bar , 33 mL EtOH and 25.1 mL (27.87 g, 422.3 mmol, 4.0 eq) of aqueous hydroxylamine solution (50 w / w%, MW: 33 g / mol, d: 1.11 g / mL). The mixture was heated to reflux temperature and stirred at reflux temperature for 5 hours. The solvent was evaporated from the reaction mixture under vacuum at 40°C. The product (IX-2) was obtained as a white solid. Yield: 84% (6.84 g). HPLC-MS (condition C) isoto...
Embodiment 2
[0357] Example 2: Synthesis of deuterated 3-(methyl-d3)-1,2,4-thiadiazole-5-carbohydrazide (I-2)
[0358]
[0359] Isotopic purity is preserved during all steps.
[0360] Step 1: Sandmeyer bromination formation (III-2-a)
[0361] At 20-25°C, put 2.7mL (4.61g Solution, containing 2.85g HBr, 35.2mmol, 3.0eq) 62w / w% HBr solution (d: 1.702g / mL), 2.1mL water and 1.39g (11.76mmol, 1eq) compound (IV-2) (isotopic purity: 93.4%, determined by titration: 85%). The mixture was heated to 40°C. Afterwards, 1.22 g (17.64 mmol, 1.5 eq) of NaNO dissolved in 2 mL of water was added through the dropping funnel 2 , the rate of addition was such that the temperature was maintained at 40-45° C. (within 5 minutes). During the addition, intense bubbling of brown gas was observed in the absorber and an oily phase separated out at the bottom of the flask. After the addition, the mixture was stirred for 1 h, monitored by TLC (Hex:EtOAc=1:1) and HPLC. The reaction mixture was cooled to 20-25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com