Composition for treatment, alleviation or prophylaxis of acne
A composition, the technology of Propionibacterium acnes, applied in the direction of drug combination, drug delivery, medical preparations containing active ingredients, etc., can solve the problem of pathogenic ribotype treatment methods without skin microbiota regulation of Propionibacterium acnes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0248] Example 1 strain screening and identification
[0249] sample
[0250] In order to identify and select bacterial strains according to the invention, a collection of lactic acid bacteria (LAB) strains was established. Samples from different sources, such as homemade sauerkraut, kimchi, and healthy human donor samples (vaginal, oral, anal, skin) were collected to isolate at least 995 species of lactic acid bacteria. Samples were collected on Man Rogosa Sharp (MRS, Sigma-Aldrich) medium and agar medium, and cultured anaerobically at 37°C overnight or until colonies formed. Isolates were plated and subcultured until pure colonies were obtained. Pure colonies were stored in MRS broth containing 25% glycerol at -80 °C for future use. Strains were identified using standard methods of 16S rRNASanger sequencing.
[0251] Example 2 - Co-aggregation
[0252] P. acnes strains obtained from BEI resources were used as research test organisms for the screening.
[0253]
[02...
example 3
[0275] Example 3 – Effect of pH on Coaggregation.
[0276] Coaggregation was determined according to the known method Cisar, J.O. et al. (1979) as described above.
[0277] All lactic acid bacteria (LAB) inoculum were grown anaerobically overnight in MRS broth, and P. acnes strains were grown anaerobically in BHI broth at 37°C to a cell density of approximately 10 8 CFU / ml (2-3 days). Overnight cell samples were harvested by centrifugation (6000 rpm for 2 minutes) and the supernatant removed from the pellet. The pellets were washed twice separately in the pH adjusted buffer used for the co-aggregation assay.
[0278] MOPS (3-(N-morpholino)propanesulfonic acid) from Merck (product 69947) and MES (2-(N-morpholino)ethanesulfonic acid) buffers from Sigma (product M2933) were used as A concentration of 100 mM was used. MES is used for pH4.5, pH5, pH5.5, pH6 and pH6.5. MOPS is used for pH6.5, pH7, pH7.5 and pH8.
[0279] Slightly less co-aggregation was found in the inert buff...
example 6
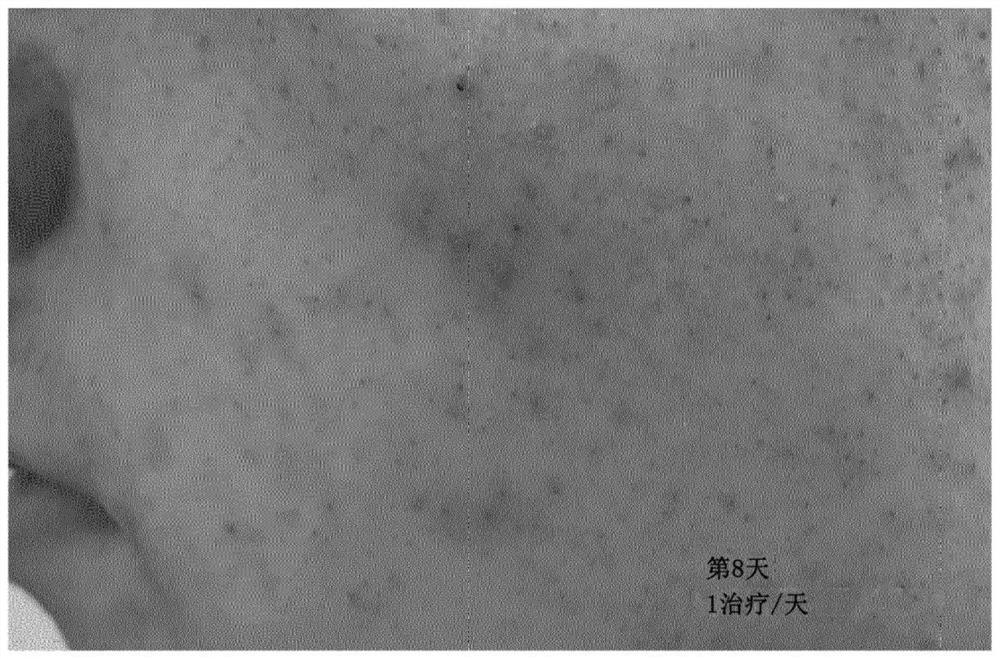
[0320] Strains LAB strain LB356R was formulated in vegetable oil containing jojoba oil and almond oil (1:1). to about 10 8 The concentration of CFU / ml oil was added to the strain.
[0321] Ten adolescents (15-19 years old) with acne-prone skin were treated with the composition once a day. Visual assessment of acne severity by pre-treatment ( Figure 1a ) and after treatment ( Figure 1b ) of the picture is OK. Visual improvement was observed in all testers and, on average, improvement was detectable after 3-4 days of treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com