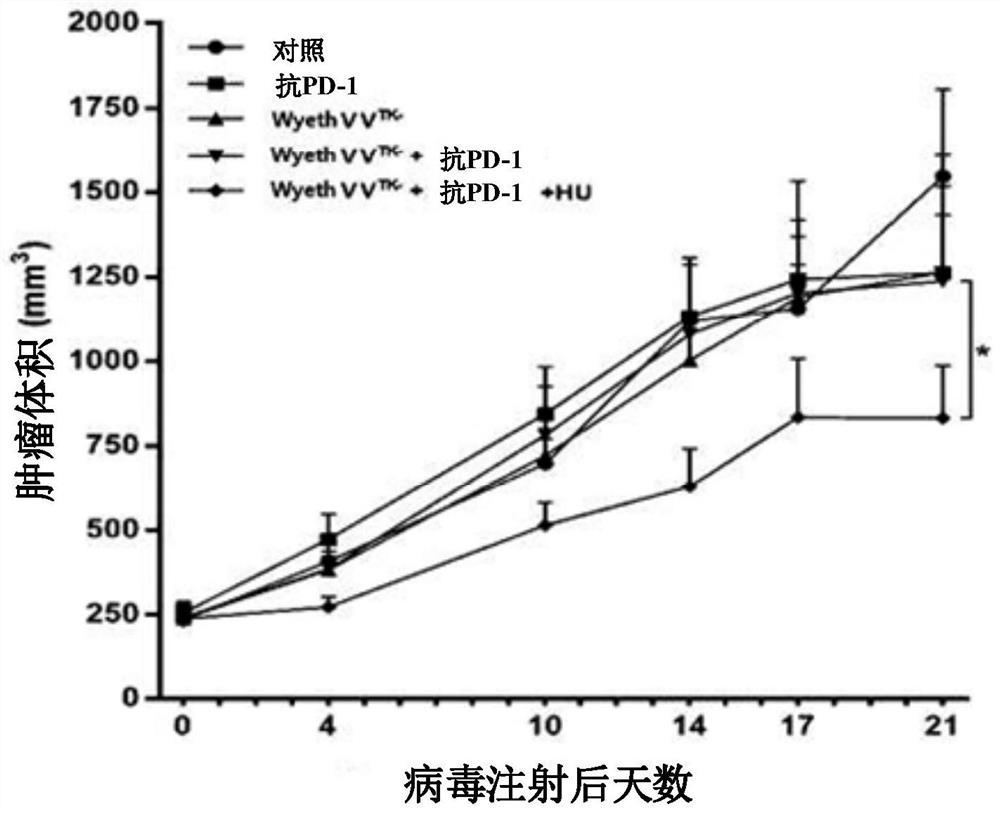
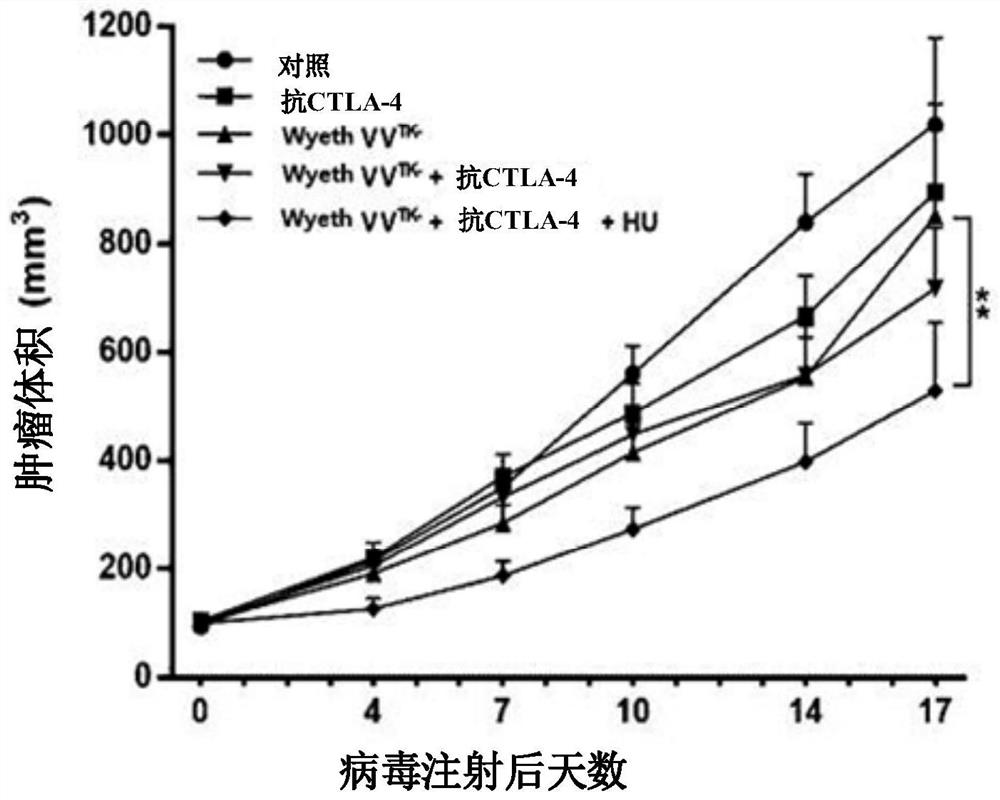
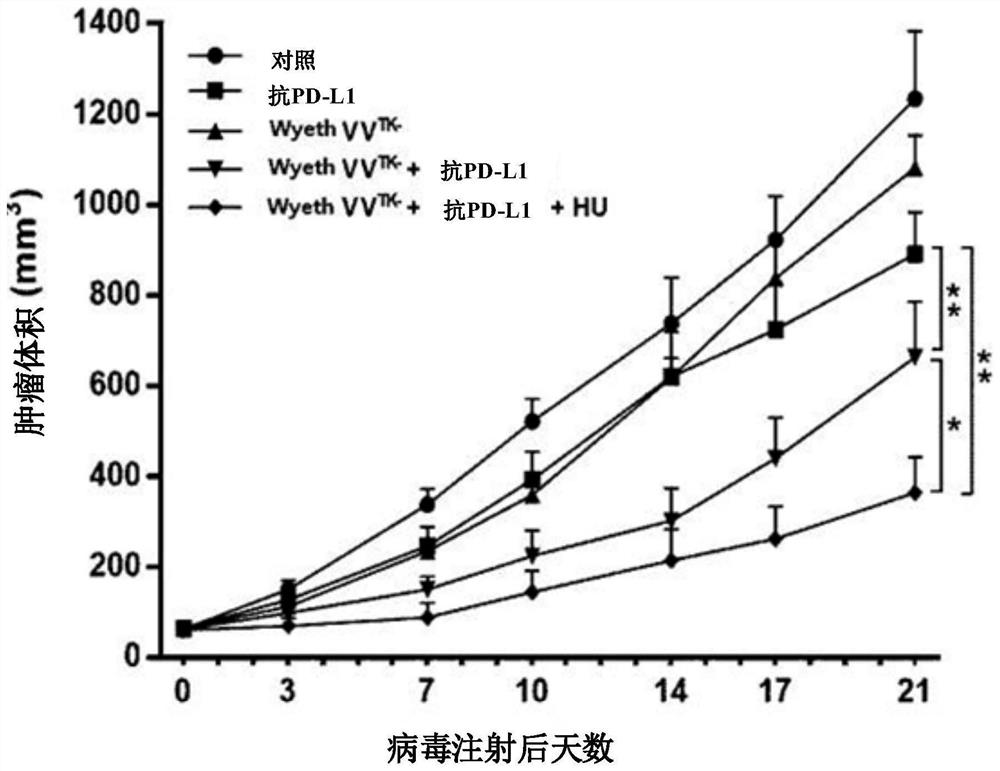
Pharmaceutical composition for treating cancer comprising anti-cancer virus, immune checkpoint inhibitor and hydroxyurea as active ingredients
A technology of immune checkpoints and active ingredients, applied in the field of pharmaceutical compositions for the treatment of cancer, can solve problems such as persistent response or complete response, progressive disease, acute tumor necrosis, etc., and achieve excellent anti-cancer effects and safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0106] Preparation Example 1. Production of oncolytic virus (Wyeth VV tk- 、WR VV tk- )
preparation Embodiment 11
[0107] Preparation Example 1.1. Construction of Shuttle Plasmid Vector
[0108] To produce oncolytic viruses lacking the thymidine kinase (TK) gene, wild-type vaccinia viruses, ie, Wyeth strain (NYC Health Department) and Western Reserve strain, were purchased from American Type Culture Collection (ATCC). For recombination, the TK region in wild-type vaccinia virus was replaced with a shuttle plasmid vector containing the firefly luciferase reporter (p7.5 promoter) gene or the GFP gene.
preparation Embodiment 12
[0109] Preparation Example 1.2. Production of Oncolytic Viruses
[0110] To obtain oncolytic virus, HeLa cells (ATCC) were grown at 4 × 10 5 Cells were seeded in 6-well plates and cultured in EMEM medium containing 10% fetal bovine serum. Subsequently, wild-type vaccinia virus was treated at an MOI of 0.05. After 2 hours, the medium was replaced with EMEM medium containing 2% fetal bovine serum, and then cells were transfected with 4 μg of the shuttle plasmid vector constructed in Example 1.1, and Xfect TM The polymer (Clonetech 631317, USA) was linearized. Incubation was carried out for 4 hours. Subsequently, the medium was replaced with EMEM medium containing 2% fetal bovine serum, and then culture was performed for 72 hours. Finally, the infected cells were collected, and then the freeze-thaw was repeated 3 times. Subsequently, the cells were lysed by ultrasound, and the free oncolytic virus was obtained by the sucrose cushion method, named Wyeth VV tk- or WRVV tk- ....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com