Methods of treating HIV with cabotegravir and rilpivirine
A technology of rilpivirine and HIV-1, applied in the direction of pharmaceutical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
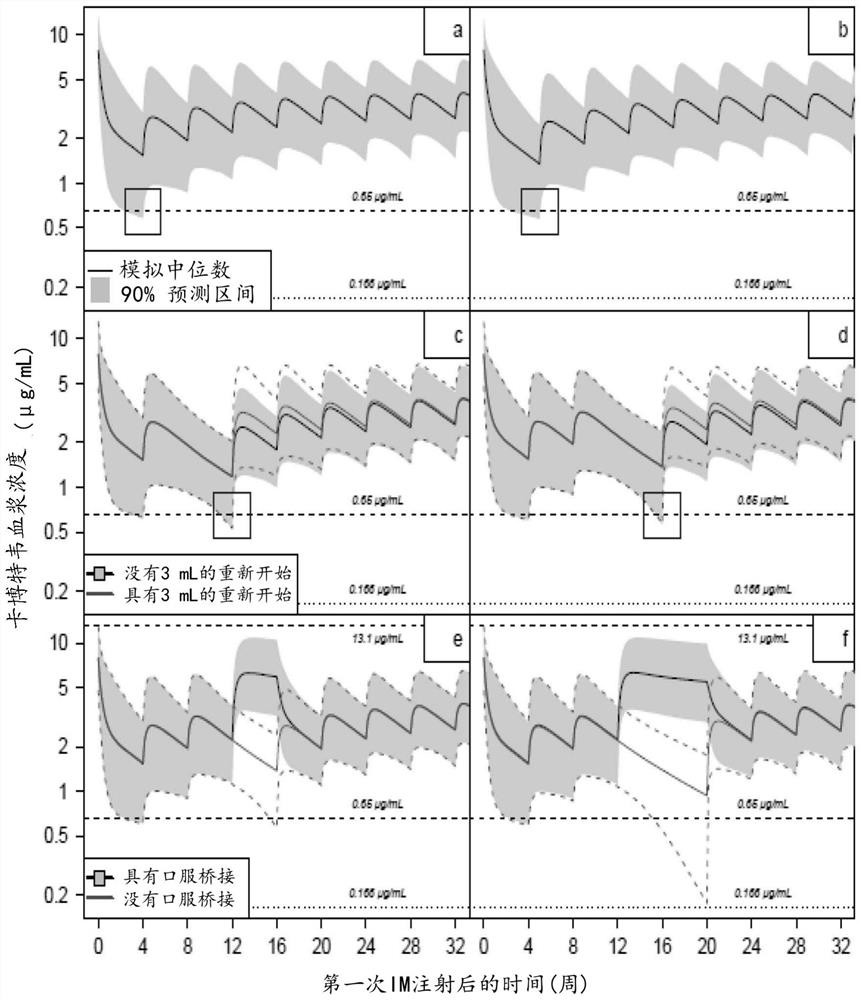
[0270] ATLAS (NCT02951052) and FLAIR (NCT02938520) are two randomized, open-label, international phase 3 studies demonstrating that switching to monthly intramuscular (IM) injections of CAB LA+RPV LA is comparable to current antiretroviral regimens (CARs) than non-inferiority. The injectable CAB+RPV LA regimen requires monthly injection visits within a prespecified time window, representing a paradigm shift for patients from daily oral dosing.
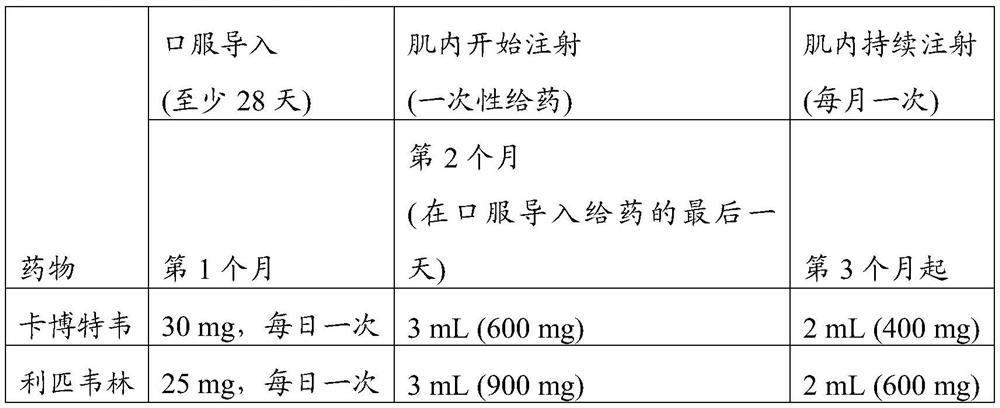
[0271] The injection is scheduled for Q4 weeks, and the planned dosing date has a dosing window of ±7 days. Adherence to LA therapy was calculated as the number of on-time injection visits that occurred within the dosing window divided by the number of expected dosing visits during the 48-week period. Both trial protocols allow for oral bridging (using oral dosing to make up for planned missed injections) to allow dosing flexibility for planned clinical point absences (such as vacation or travel) while allowing subjects to maintain LA...
Embodiment 2
[0281] A two-compartment model with first-order oral and intramuscular (IM) absorption and first-order elimination adequately described data from 23,926 concentration records in 1647 subjects following oral and LA administration (Han K, Patel P, Baker M et al Human, Populationpharmacokinetics of Cabotavir in adult healthy subjects and HIV-1 infected patients following administration of oral tablet and long acting intramuscular injection. Abstract WEPDB0205.22nd International AIDS Conference23–27July 2018, Amsterdam, the Netherlands).
[0282] Covariates retained in the model included gender, BMI, needle length, and fractional injection of the absorption rate constant (KA LA) after LA administration, as well as the effect of current smoker status and body weight on CL and volume. No CAB dose adjustment was required for the covariate assessed.
[0283] In the Phase 3 study, the 5th percentile of Individual Predicted Concentration (IPRED) at the postloading trough (0.65 μg / mL) fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com