Pharmaceutical formulations and systems for delivery of androgen agents and aromatase inhibitors and methods of use
A technology of aromatase inhibitors and pharmaceutical preparations, which is applied in drug combination, drug delivery, and pharmaceutical formulations, and can solve problems such as side effects and decreased serum estradiol levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
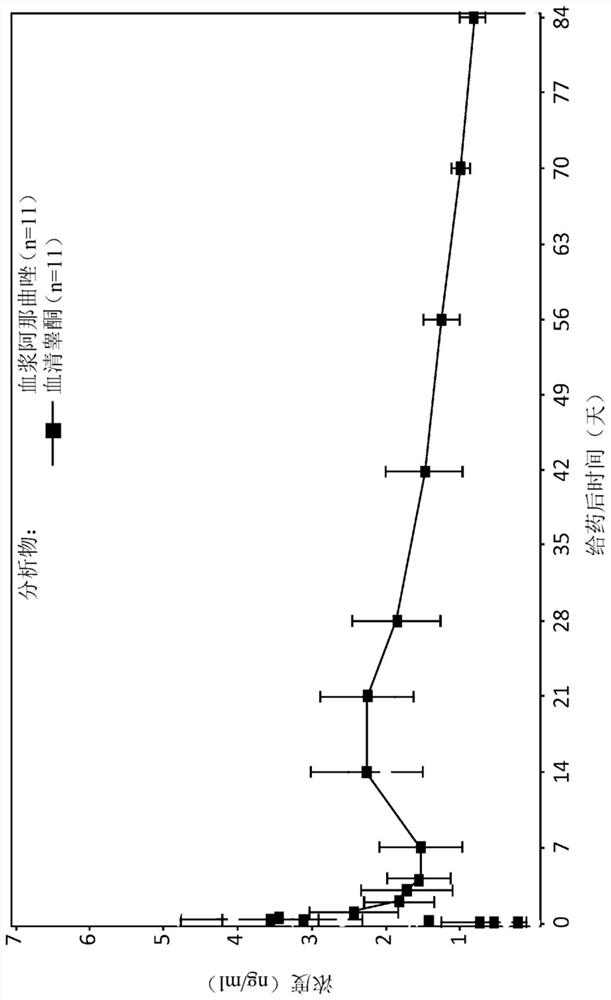
[0304] Example 1 : Single-dose trial to evaluate the pharmacokinetics of testosterone and anastrozole from subcutaneous testosterone and anastrozole implants (80 mg / 4 mg (T+Ai)) in women
[0305] Pharmacokinetic analysis was performed on subcutaneous testosterone 80 g and anastrozole 4 mg implants in 11 healthy volunteers with high mammographic breast density. This study evaluated the pattern of both anastrozole and testosterone and allowed modeling of the pharmacokinetics of this exemplary implant to demonstrate the pattern and end-organ pharmacodynamics known to exist in excess of aromatase activity Response, ie, end-organ pharmacodynamic response in tissue of women with high mammographic breast density. This is assessed by direct measurement of breast tissue elasticity in relation to mammographic breast density.
[0306] Ultrasonic evaluation of pellet dissolution was performed to demonstrate how to perform a comprehensive analysis of infusion dissolution of reproductive h...
example 2
[0334] Example 2: Testosterone and Anastrozole Combination Therapy for MBD Reduction in Women
[0335] This example provides an analysis of the use of testosterone and anastrozole combination therapy to reduce mammographic breast density (MBD) in women. The study was conducted at the Wellend Clinic (Burnside War Memorial Hospital, Adelaide, South Australia). The main indications for therapeutic intervention are one or more of the following: perimenopausal hormonal dysfunction, high MBD, which is considered a factor in reducing breast cancer (BC) risk.
[0336] The 652 patients were all female, and the mean age at first T+Ai implantation was 52 years (range, 23 to 79 years).
[0337] MBD / BC risk reduction was the primary indication for treatment in 89 patients (14%), adjunctive hormonal dysfunction in 334 patients (51%), and both indications in 177 patients (27%) . Fifty-two patients did not provide primary indications.
[0338]A history of BC was noted in 90 patients (14% ...
example 3
[0394] Example 3: Pharmacokinetic profile of anastrozole using a modeling approach
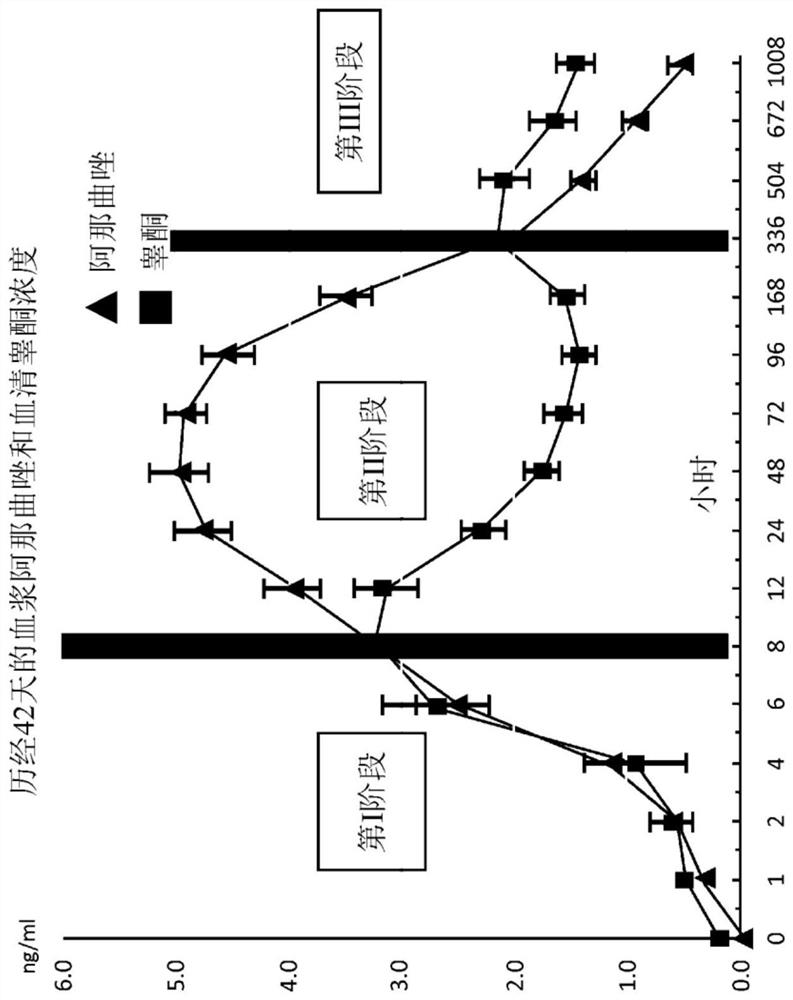
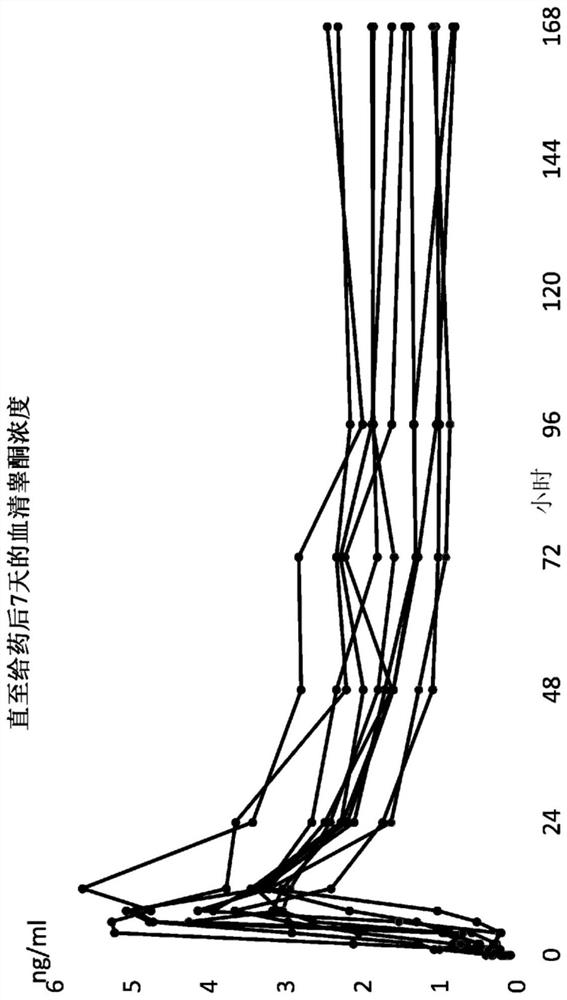
[0395] This example provides an analysis of the pharmacokinetic profile of anastrozole using a modeling approach to evaluate the best input function describing the absorption of the assay described in Example 1. Table 6 lists the sampling schedule for anastrozole and testosterone. At least 8 mL of blood samples were collected by venipuncture for serum testosterone / DHT and plasma anastrozole at each time point indicated in Table 6. Figure 7A-B Anastrozole absorption rate is described. Data in these figures are expressed as mean + / - standard deviation. Figure 7A shows the Y-axis on a log scale. Figure 7B shows the Y-axis on a linear scale. This number was generated using the pellet absorption data summarized in Table 2 of Example 1. The percent change from baseline in mean volume was calculated and plotted against time in months. Figure 8A -B plots post-implantation plasma anastrozole co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Bmi | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap