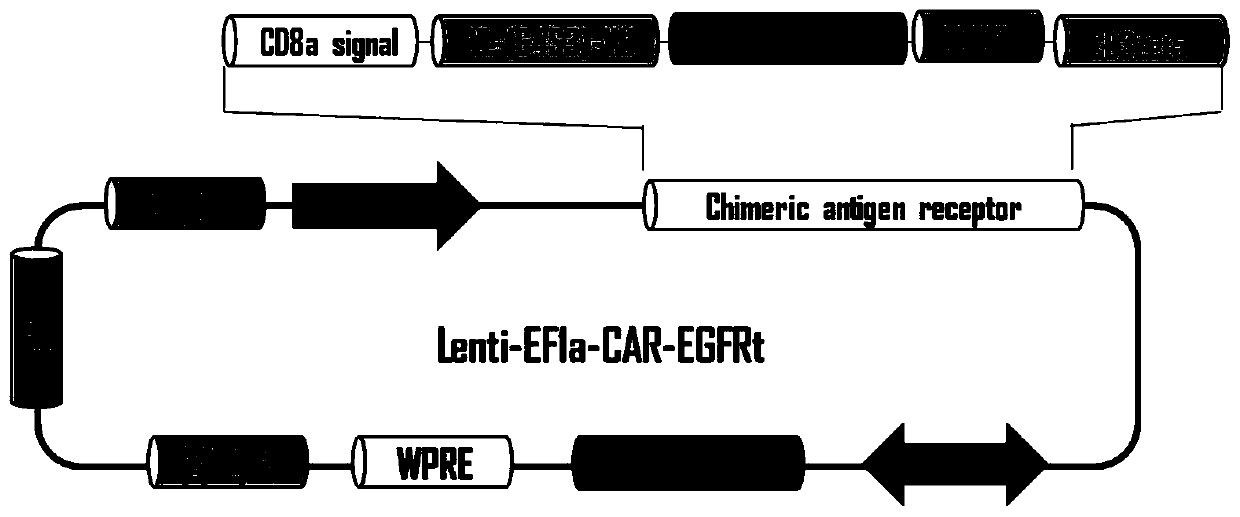
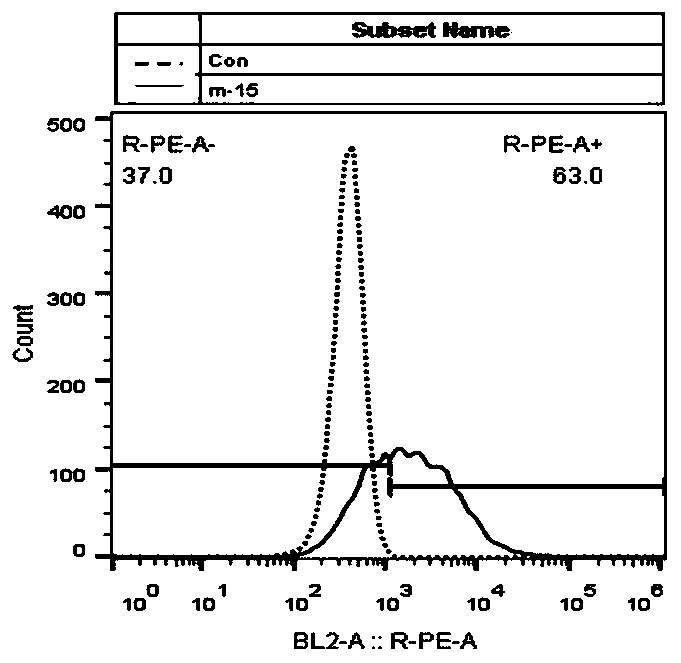
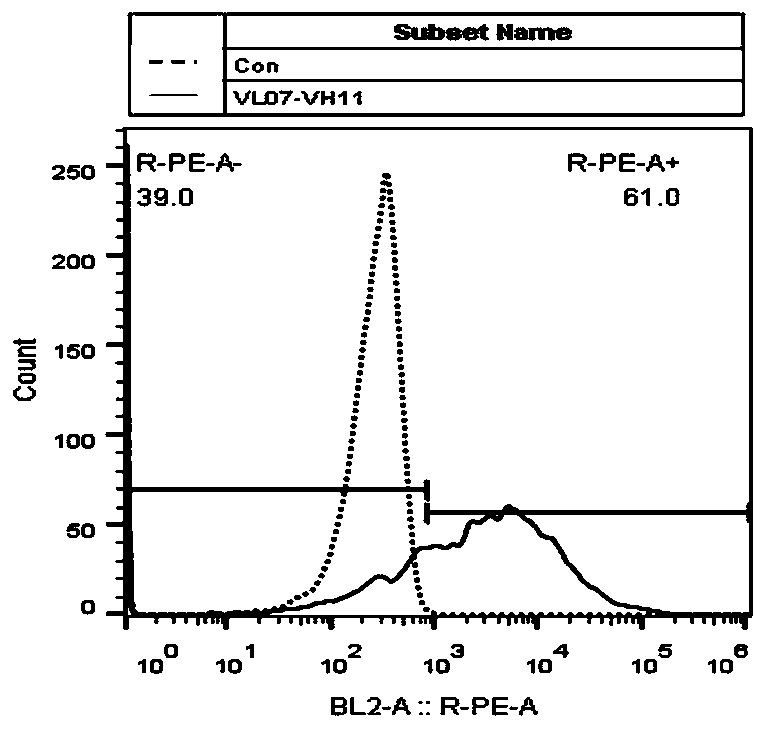
Chimeric antigen receptor and application thereof
An antibody and antigen technology, applied in the field of medicine and biology, can solve the problem of unsatisfactory effect of immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0557] Example 1: Production of anti-BCMA murine antibody
[0558] 1) Antigen preparation
[0559] The CDS sequence of human BCMA (NM_001192.2) was retrieved from NCBI data. After the whole gene was synthesized, it was cloned into the vector; CHO-K1 (ATCC) was revived and cultured to keep the cells in the logarithmic growth phase. The cells were infected with lentivirus to overexpress BCMA, and the CHO-K1-BCMA recombinant cell line with high expression of BCMA protein was constructed. A K562 (ATCC)-BCMA cell line with high expression of BCMA was screened by a similar method.
[0560] 2) immunity
[0561] Resuscitate the CHO-K1-BCMA cell line expressing the target antigen, take 5-8 Balb / C mice, inject the CHO-K1-BCMA cell suspension expressing the target antigen into the abdominal cavity of the mouse with a disposable syringe, and repeat the immunization three times ; Carry out booster immunization and hybridoma preparation.
[0562] 3) SP2 / 0-B cell fusion
[0563] The mye...
Embodiment 2
[0566] Example 2: Affinity Determination of Anti-BCMA Murine Antibody
[0567] Surface plasmon resonance (SPR) can dynamically reflect the association / dissociation rate and affinity constant of antibody-antigen interaction in real time. If the sample to be assayed is an antibody, it can be directly carried out with the cultured supernatant of hybridoma cells. Its operation process is in the surface plasmon resonance (SPR) biosensor ( T200 (GE HealthCare)), in short, firstly, the antigen (human BCMA) recognized by the antibody to be tested (the monoclonal antibody secreted by the hybridoma obtained in Example 1) was coupled to the surface of the sensor chip CM5, Then the antibody to be tested is injected, and the antibody-antigen binding process is directly monitored through the chip surface, and the kinetic parameters of the fast semi-quantitative antibody to be tested can be measured. The results showed that the binding rate constant of the mouse monoclonal antibody was ka...
Embodiment 3
[0568] Example 3: Humanization and affinity determination of anti-BCMA murine antibody
[0569] 1) Determination of the sequence of the variable region of the murine antibody
[0570] Take 1×10 6 The hybridoma monoclonal cells obtained in Example 1 were subjected to RNA extraction, and cDNA was prepared, and VH and VL were cloned and sequenced to obtain the heavy chain variable region sequence SEQ ID NO: 1 and the light chain variable region sequence of the murine antibody. The sequence of the variable region is SEQ ID NO: 2; the heavy chain CDRs (HCDR1, HCDR2 and HCDR3) of the antibody are SEQ ID NOs: 3-5 respectively, and the light chain CDRs (LCDR1, LCDR2 and LCDR3) of the antibody are SEQ ID NOs: 6-8. The above CDRs region sequences are defined by the Chothia numbering system, and any other method for determining the CDR region sequence known in the art can also be used to identify the amino acid residues of the CDR region in the variable region.
[0571] 2) Design and p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com