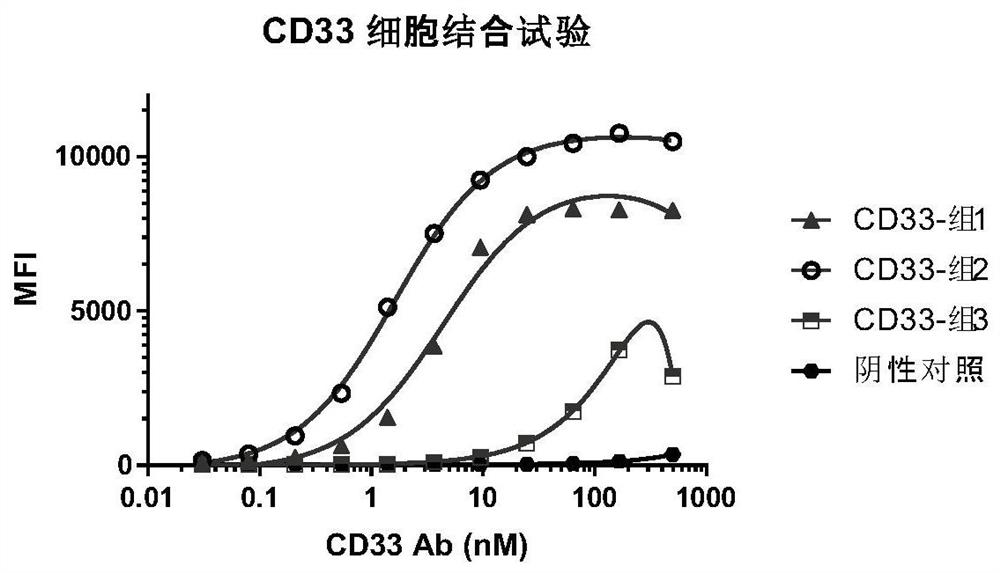
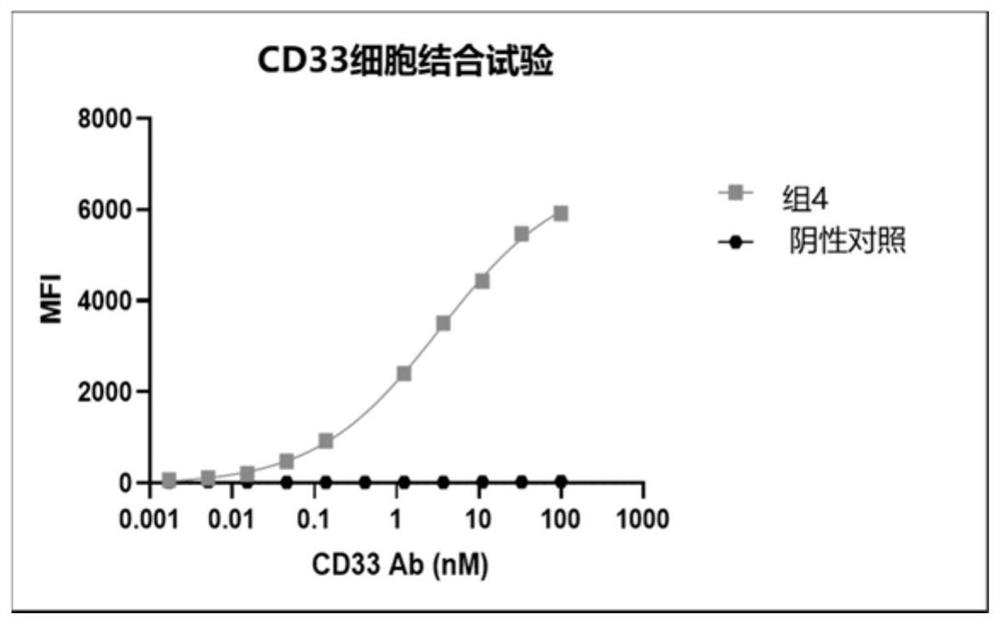
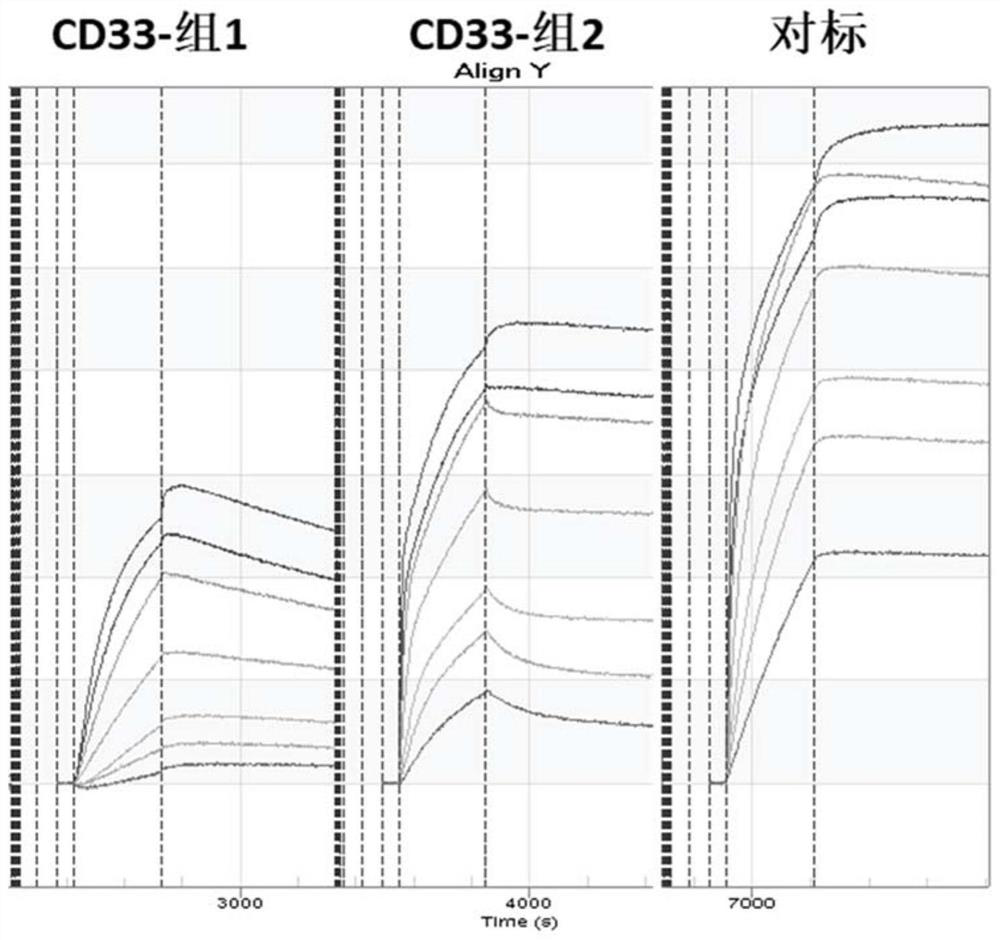
Chimeric antigen receptor specifically binding to CD33 and application thereof
A specific, CDR-H3 technology, applied in the direction of application, antibody, cancer antigen components, etc., to achieve significant clinical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0314] Example 1: Preparation of specific single-chain antibody (scFv) binding to human CD33
[0315] 1.1 Screening of CD33 scFv based on phage display
[0316] (1) Take 0.5ml of a fully human phage library and screen it with biotinylated CD33 and SV magnetic beads, and the screened products are tested for phage titration by plating.
[0317] (2) Mix 0.5ml of the product of the first round of panning with 0.5ml of M PBST, and carry out the second, third or fourth round of panning according to the above steps.
[0318] 1.2 ELISA detection of monoclonal phage
[0319] Inoculate a single colony from the phage panning product titer plate into a 96-deep well plate, and detect monoclonal phage by ELISA.
[0320] 1.3 CD33-scFv sequencing
[0321] The forward and reverse primers used for sequencing were: PKLT1F (SEQ ID NO: 81); PKLT1R: (SEQ ID NO: 82). The sequence results were analyzed using Sequcher software, and finally 11 clone sequences were obtained.
[0322] 1.4 Constructi...
Embodiment 2
[0340] Example 2: Construction of lentiviral plasmid and viral packaging
[0341]1) Overview of the construction of the lentiviral plasmid: the scFv sequence was synthesized after codon optimization and constructed into the Lenti-EF1a-AT-Free (manufactured by Suzhou Aikangde Co., Ltd.) vector, and a single clone was picked for cultivation and preservation, and the plasmid was extracted Sequencing is performed, and the bacterial liquid with correct sequencing is used to prepare lentiviral plasmids.
[0342] In detail: based on the scFv sequence obtained in the above examples, further construct the CAR lentiviral expression vector. Using the intracellular domain of CD137 and the ITAM region of CD3ζ as the activation signal, fuse with the above scFv, add signal peptide, CD8 hinge region, and CD8 transmembrane region at the same time, construct a chimeric antigen receptor expression vector, and subclone it into In the Lenti-EF1a-AT-Free (manufactured by Suzhou Aikond Co., Ltd.) v...
Embodiment 3
[0348] Example 3: Preparation of CAR-T cells
[0349] 3.1 Isolation and activation of primary T cells:
[0350] (1) Use lymphocyte separation medium to separate human PBMC cells, add totipotent nuclease according to the ratio of cell fluid:totipotent nuclease=10ml:1ul, place at 37°C, 5% CO 2 Cultivate for 3 hours in an incubator;
[0351] (2) Aspirate CD3 / CD28 magnetic beads according to the ratio of PBMC: magnetic beads = 3:1, wash with HDPBS (DPBS containing 2% human albumin), and resuspend in 100ul HDPBS in a cryopreservation tube.
[0352] (3) Collect the suspended cells in a cryopreservation tube, centrifuge at 500g for 5 minutes, add 1ml HDPBS to resuspend the cells, transfer the cell suspension to 100ul magnetic beads, mix gently, and incubate at room temperature for 30 minutes;
[0353] (4) Insert the incubated mixture into the magnetic pole, let it stand at room temperature for 2 minutes, keep the cryopreservation tube in the magnetic pole, invert it gently, and pou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com