Anti-PD-1 antibody and application thereof
A PD-1 and antibody technology, applied in the field of pharmaceutical compositions containing them, can solve the problems of weakening the clinical therapeutic effect and universality of PD-1 antibodies, toxic and side effects, therapeutic doses, limitations, etc., to improve blocking activity And the effect of inducing T cell activation, high degree of humanization, and significant clinical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0459] Example 1: Production of mouse-derived anti-PD-1 monoclonal antibody
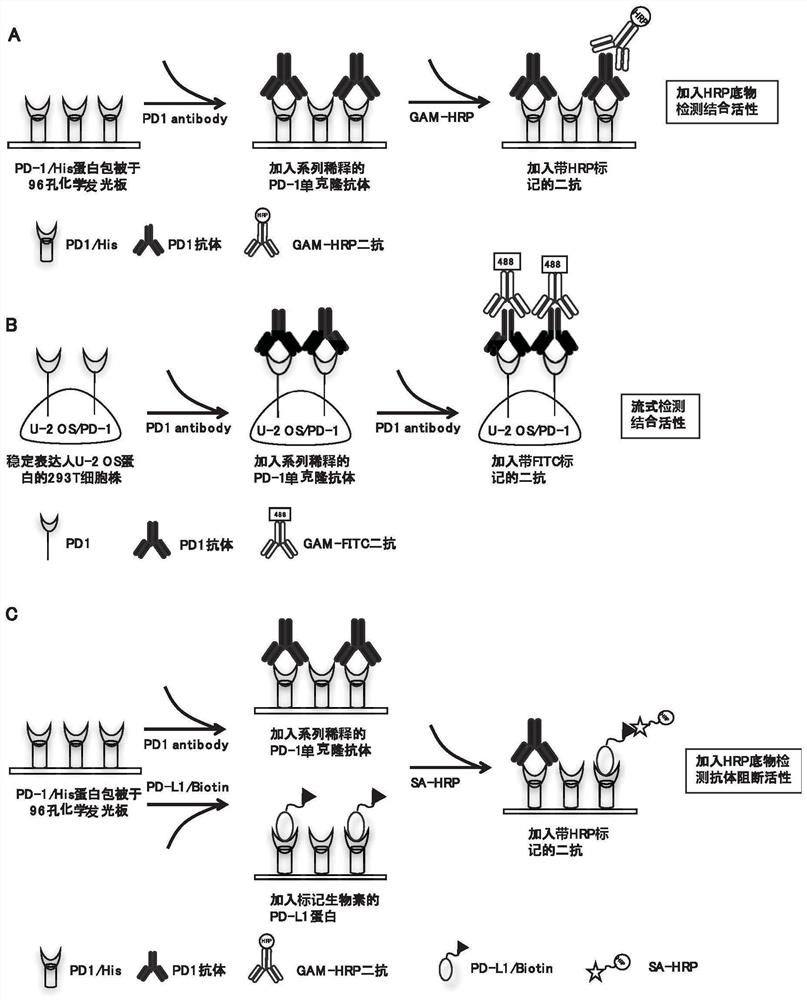
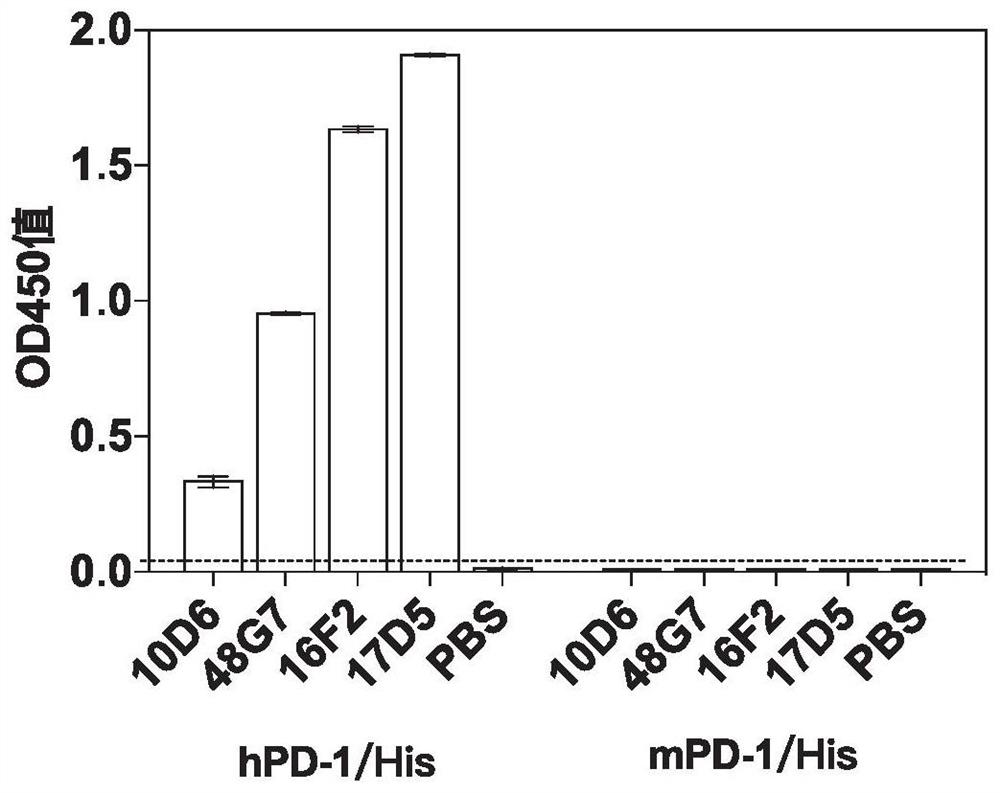
[0460] In this example, the anti-human PD-1 monoclonal antibody was obtained by conventional hybridoma fusion technology; the monoclonal antibody with high binding activity was screened by enzyme-linked immunosorbent assay (ELISA) and flow cytometry (FACS) ; Use chemiluminescent enzyme-linked immunoassay (CLEIA) to screen monoclonal antibodies that can highly block the interaction between PD-1 and PD-L1.
[0461] 1.1 Construction of PD-1 expression plasmid
[0462] The human full-length PD-1 sequence (genbank: NM_005018.2) was amplified by PCR using the primers shown in Table 2, and ligated into the plv vector (Addgene Company) to construct a plasmid expressing PD-1, named for plv-PD-1.
[0463] In addition, a plasmid expressing human full-length PD-L1 (named plv-PD-L1) was obtained by the same method. For the sequence of the human full-length PD-L1, see genbank: NM_014143.3.
[0464] Table 2: Pri...
Embodiment 2
[0487] Example 2: Characterization of murine anti-PD-1 monoclonal antibody
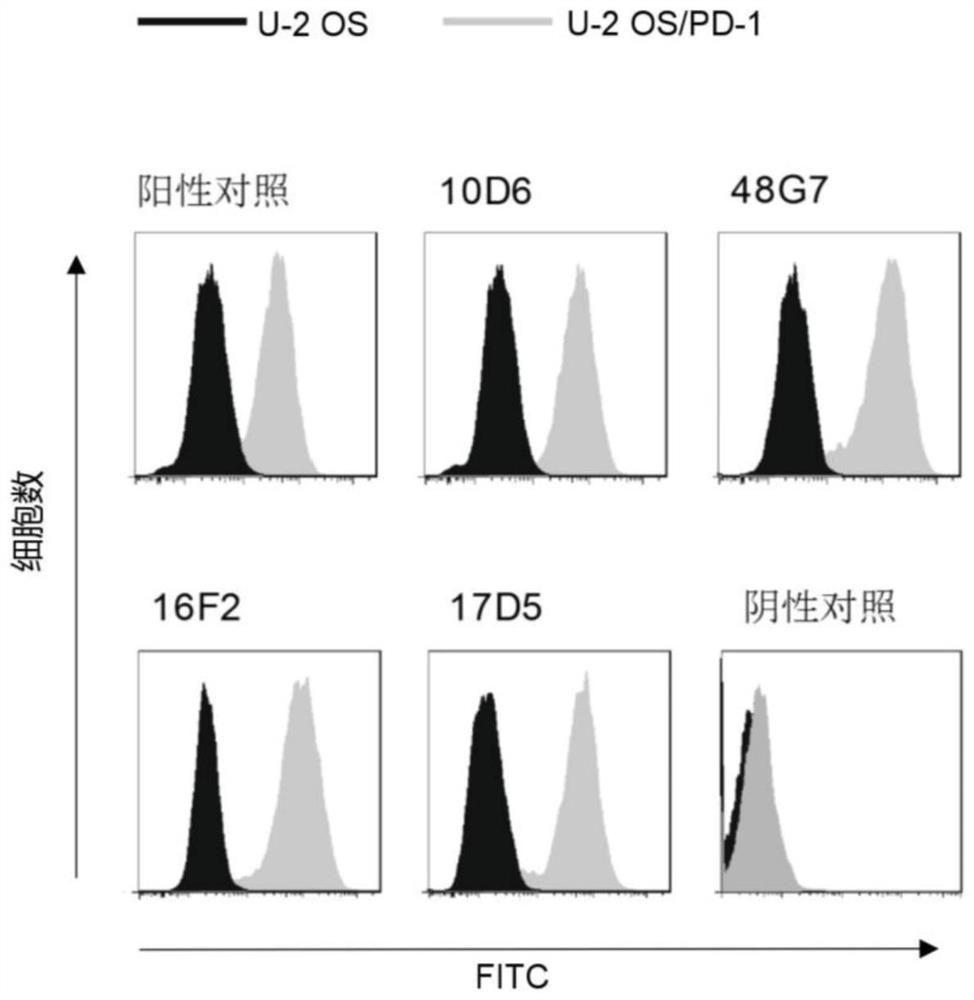
[0488] 2.1 Detection of PD-1 antibody binding activity by FACS method
[0489] The principle of FACS method to detect the binding activity of PD-1 antibody is as follows: figure 1 Shown in B.
[0490] The U-2OS / PD-1 cells to be tested were digested and blown to make a single cell suspension, which was resuspended in PBS containing 3% fetal bovine serum after centrifugation. The cell suspension was filtered through a 200-mesh screen for testing, and each cell sample was collected about 1×10 5 cells. Dilute the antibody to be tested to 1 μg / mL and incubate with the above cells, use FITC-labeled goat anti-mouse IgG antibody (Sigma Company, product number F9006) and use flow cytometry in a flow cytometer (BD Company, FACS Aria II ) to detect the binding activity of the PD-1 antibody. The abscissa represents the fluorescence value of the detected cells.
[0491] The result is as image 3 As show...
Embodiment 3
[0496] Embodiment 3: surface plasmon resonance method (SPR) detects the antigen affinity activity of antibody
[0497] Antigen affinity determination of antibodies was carried out using BIAcoreTM instrument (GE Healthcare company, model BIACORE3000). Anti-mouse Fc CM5 biosensor chips (GE Healthcare) were generated using standard primary amine coupling procedures. The murine antibody was captured on the anti-mouse Fc surface for 1 min at a flow rate of 10 μl per min. Serial dilutions of PD-1 / Fc from 3.3 nM to 120 nM were injected on the antibody-binding surface at a flow rate of 30 μl per minute for 3 minutes, followed by a 10-minute dissociation phase. Use the EIA evaluation software (GE Healthcare company) one-to-one Langmuir (Langmuir) binding model to calculate binding rate (K a or K on ) and dissociation rate (K d or K off ). at a rate of K off / K on to calculate the equilibrium constant (K D ). The results are shown in the table below. The results showed that 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com