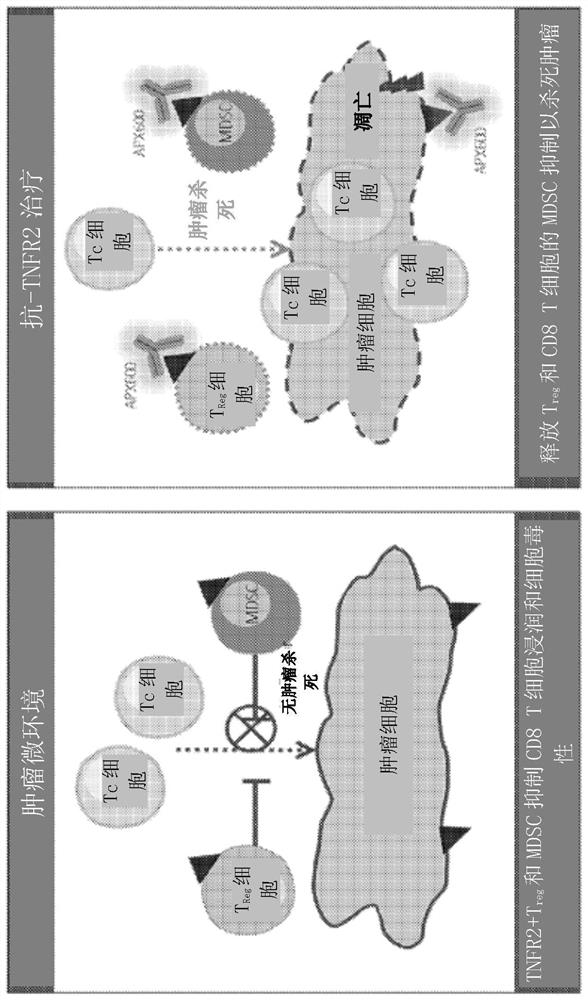
Anti-TNFR2 antibodies and methods of use thereof
A technology of antibodies and receptors, applied in the direction of antibodies, chemical instruments and methods, anti-tumor drugs, etc., can solve problems such as reducing and hindering signal transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0391] Immunization and initial screening
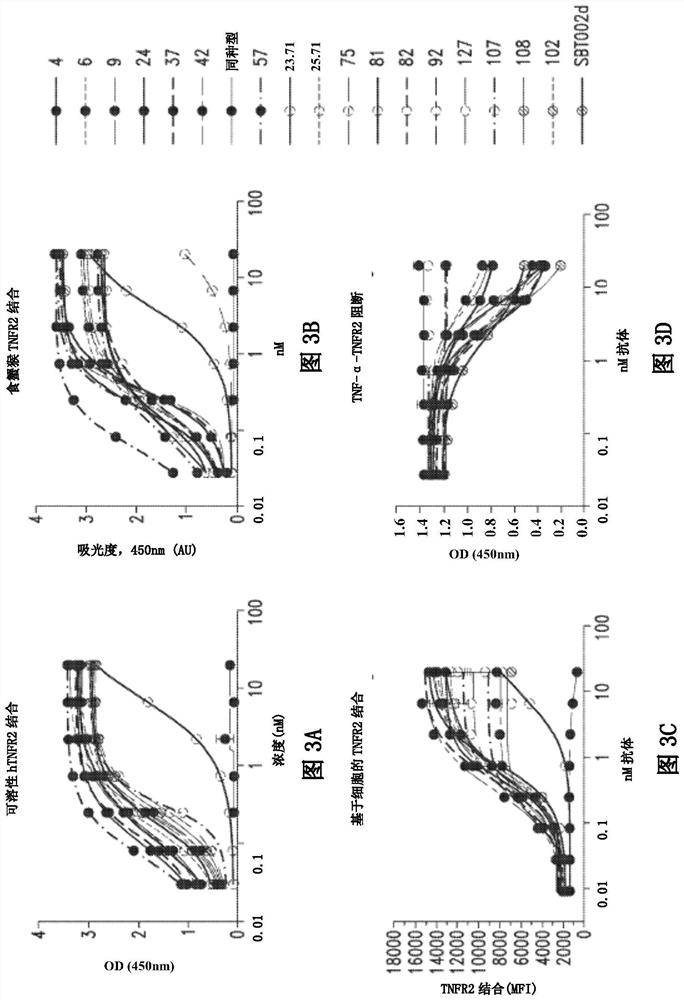
[0392] To prepare antibodies for screening, four New Zealand white rabbits were immunized and subsequently boosted with human 293-TNFR2 overexpressing cells or human TNFR2-Fc fusion protein. All rabbits had serum titers specific for human TNFR2 and were used for the use of APXiMAB TM Technology Generation of hybridomas and antibody production by B cell culture method (RevMAb). After initial screening, 460 antibodies were identified that bound to CHO-TNFR2 overexpressing cells. Screening processes to identify potential lead candidates such as figure 2 shown.
[0393] Supernatants from hybridoma and B cell cultures were then screened for antibodies that could block the binding of TNF-α to soluble TNFR2-His (Sino Biological; 10417-H08H) in an ELISA-based receptor ligand binding assay. Of the 460 antibodies identified, 173 showed inhibition of TNF-α binding to TNFR2, which was greater than 75% of maximal binding.
[0394] 110 antib...
Embodiment 2
[0405] Binding and activity characterization of clones h600-25-71 and h600-25-108
[0406] The binding and activity characteristics of humanized clones h600-25-71 and h600-25-108 were tested. To test binding to TNFR family members, target proteins were coated on ELISA plates at 1 μg / mL overnight at 4°C. Plates were washed, blocked, and antibodies were added at the indicated concentrations for 1 hour at room temperature. Positive control antibodies for each protein were used at approximately 1 ug / mL. Antibodies were washed and detected with anti-human IgG HRP for 1 hour. The assay was developed using TMB substrate for 10 minutes. For cell binding, human CD4+ T cells purified from buffy coat were incubated with anti-CD3 / CD28 in flat bottom plates for 24 hours. Cells were harvested and stained for viability, CD4, CD25 and FOXP3. Stain with anti-human IgG APC detection test antibody. Binding titers of test antibodies were assessed on CD4+CD25hiFOXP3+ regulatory T cells and p...
Embodiment 3
[0418] Identification of the epitope site of clone 25-71
[0419] Experiments were performed to determine the epitope of the TNFR2 / 25-71 complex at high resolution.
[0420] First, high-quality MALDI analysis was performed on individual TNFR2 samples and individual clone 25-71 samples to verify integrity and aggregation levels. Measurements were performed using an Autoflex II MALDI ToF mass spectrometer (Bruker) equipped with an HM4 interaction module (CovalX) containing a detection system designed to optimize detection up to 2 Mda with nanomolar sensitivity. The TNFR2 sample powder was dissolved in distilled water to a concentration of 1 mg / ml and 20 μl of each protein sample of TNFR2 and clones 25-71 were pipetted to prepare 8 dilutions with a final volume of 10 μl. Then, 1 μl of each dilution was mixed with 1 μl of matrix consisting of recrystallized sinapic acid matrix ( 10mg / ml) composition. After mixing, 1 μl of each sample was spotted on MALDI plates (SCOUT 384). Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com