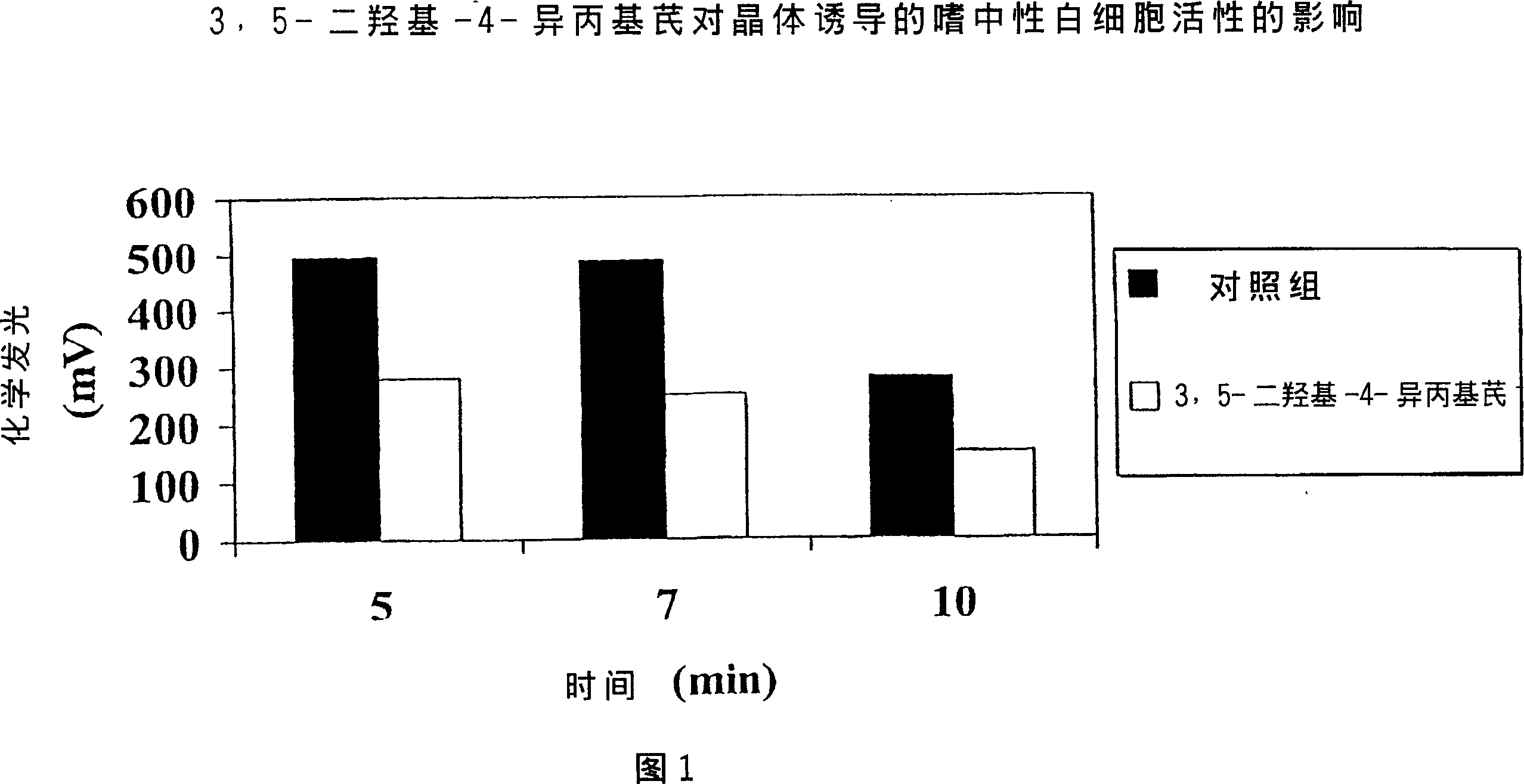
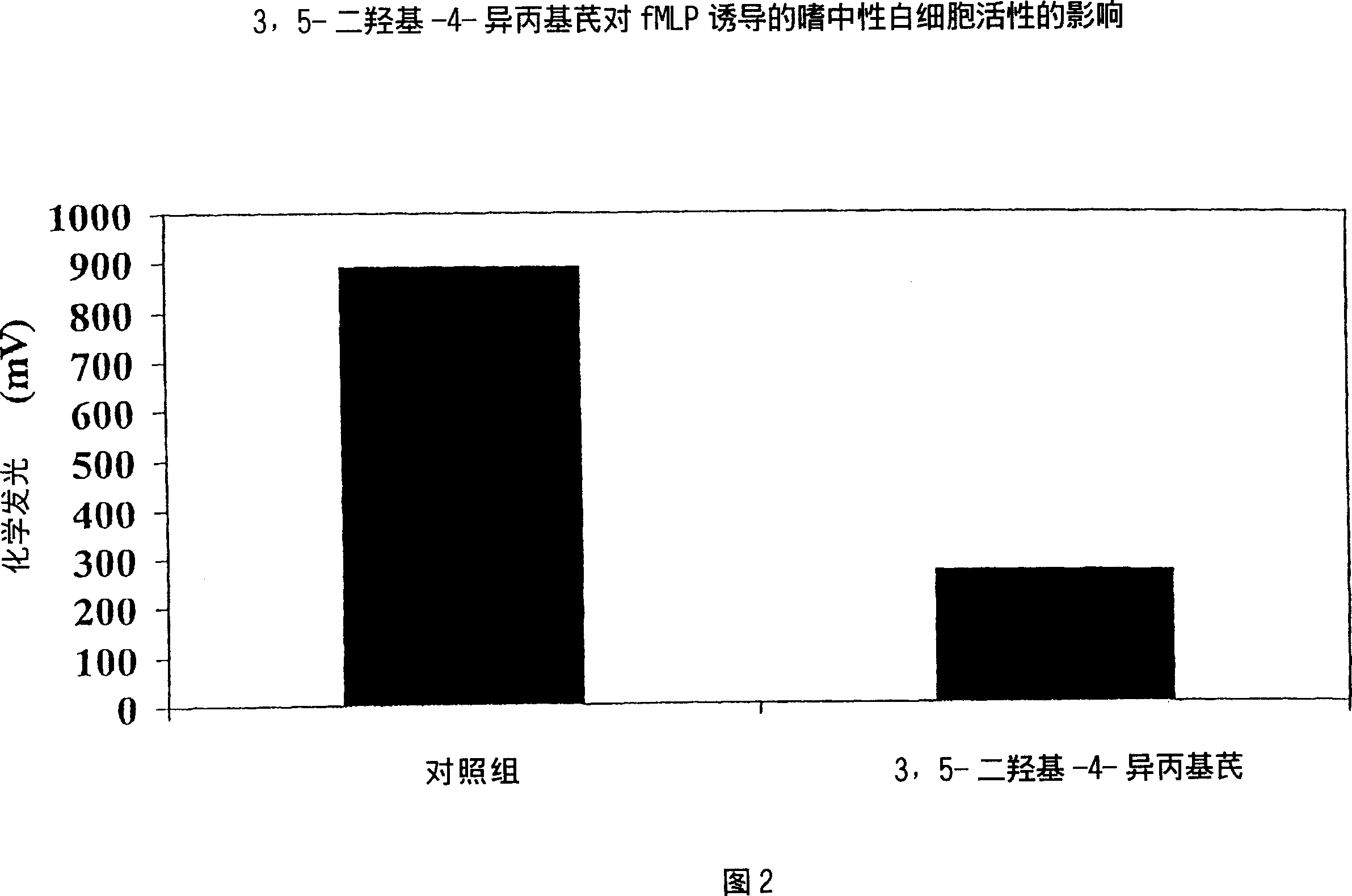
Polyhydroxystilbenes and stibene oxides as antisoriatic agents and protein kinase inhibitors
A technology of dihydroxy and propyl stilbene, which is applied in the fields of anti-inflammation, treatment of psoriasis and inhibition of protein activating enzymes, hydroxy stilbene, stilbene derivatives and their analogues, which can solve the problems of erythrocyte giant cells, lack of correlation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1-synthesis of compounds of the present invention
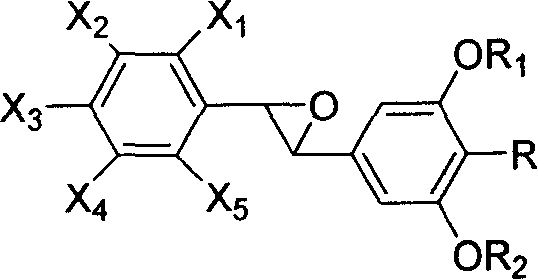
[0052] Compounds useful in the compositions of the present invention can be prepared from 3,5-dihydroxybenzoic acid and 4-bromo-3,5-dihydroxybenzoic acid of the formula
[0053]
[0054] Synthetic methods include hydroxymethylation, reduction of esters, oxidation of alcohols, witting reactions (or Horner reactions or Horner-Emmons-Wadsworth reactions) and demethylation reactions. This synthetic route is well established and available in the art.
[0055] Other stilbene derivatives can be obtained by reaction of hydroxylated stilbene derivatives with an acid or derivatives thereof, such as the corresponding salts, chlorides and anhydrides, by standard esterification reactions. Such reactions are well known in the art. For example, an alcohol is added to a mixture of anhydride and pyridine at low temperature and the mixture is left at room temperature for a time sufficient to complete the reaction. After...
Embodiment 2
[0112] Example 2 - Formulation of Compounds of the Invention
[0113] Element
quantity
Active ingredient (compound of the invention)
0.05-20.0mg
ethanol
100μl
Mineral oil, USP
50.0mg
White petrolatum, USP
made into 1.0g
[0114] step
[0115] Heat the weighed white petrolatum and mineral oil to 65°C and mix well. The mixture was cooled to 50-55°C with stirring. Certain active ingredients, dissolved in ethanol and ground, are added to the above mixture with stirring. The ointment was cooled to room temperature.
[0116] Element
quantity
Active ingredient (compound of the invention)
0.05-20.0mg
ethanol
100μl
Micronized aluminum monostearate
50.0mg
Isopropyl myristate
made into 1.0g
[0117] step
[0118] About 90% of the desired isopropyl myristate was heated to 60°C. Add the aluminum monostearate with stirring and keep heating to ...
Embodiment 3
[0125] Example 3 - Application as an antipsoriatic agent
[0126] Since there is no suitable animal model for this disease, the antipsoriatic activity of the compounds of the present invention can be determined by testing the effect of the compounds in vivo. The compound was tested by volunteers. In these tests, a representative compound of the invention, 3,5-dihydroxy-4-isopropylstilbene, showed activity in reducing or eliminating psoriasis symptoms.
[0127] The composition of the present invention comprises an antipsoriatic amount of a compound of formula I or formula II, and a suitable pharmaceutical carrier. An antipsoriatic amount is defined as the amount of compound necessary to eliminate psoriatic symptoms, ie psoriatic lesions. In the usual course of treatment, the active compound is incorporated into an acceptable carrier to form a composition for topical administration to the affected area, or into a composition suitable for oral administration. in the form of ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com