Catalyst for removing carbon monoxide in hydrogen rich gas according to water gas shift reaction ,processing device and method using the catalyst
A carbon monoxide and catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve proton exchange membrane fuel cell damage, catalyst CO conversion rate decline, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
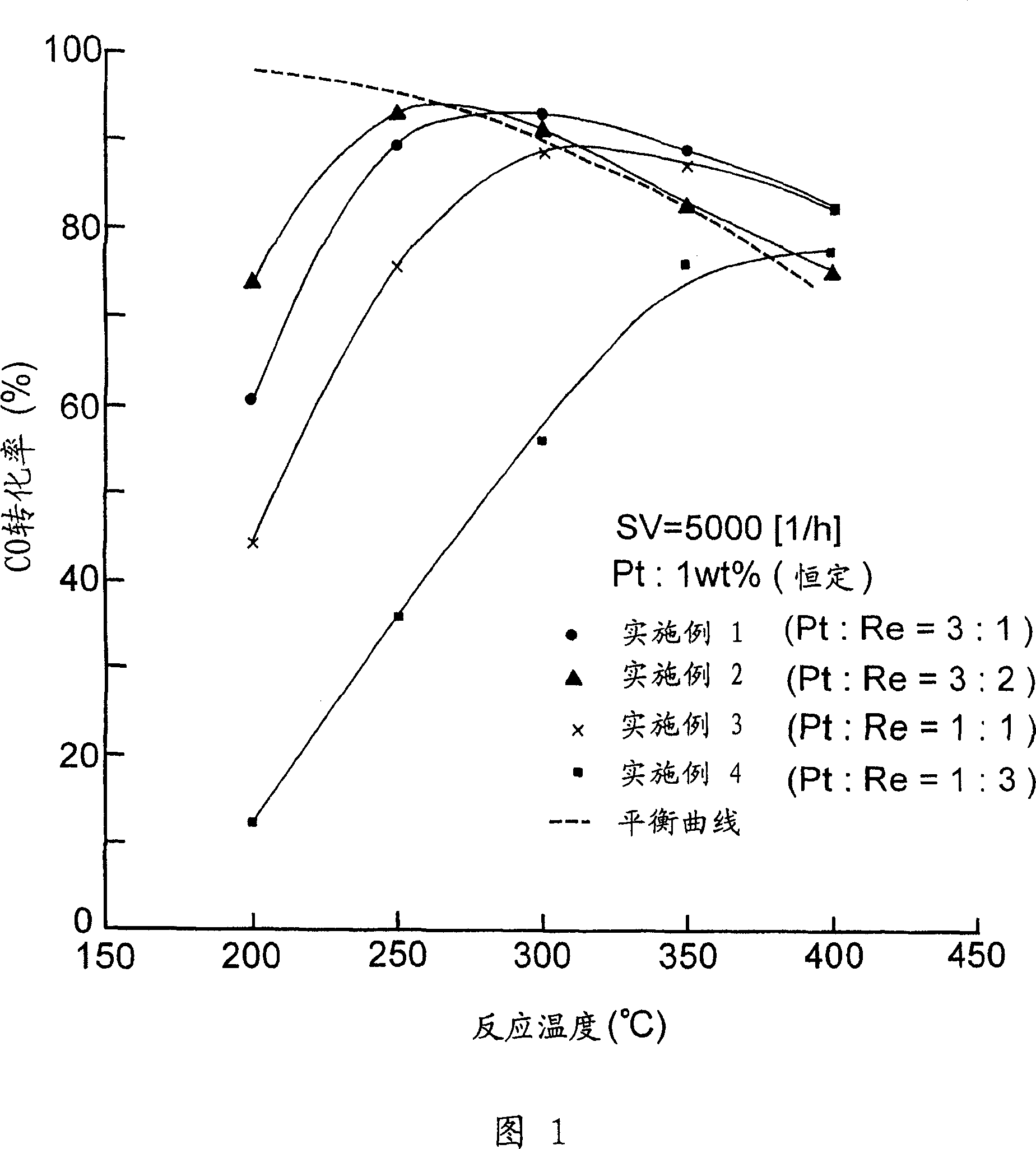
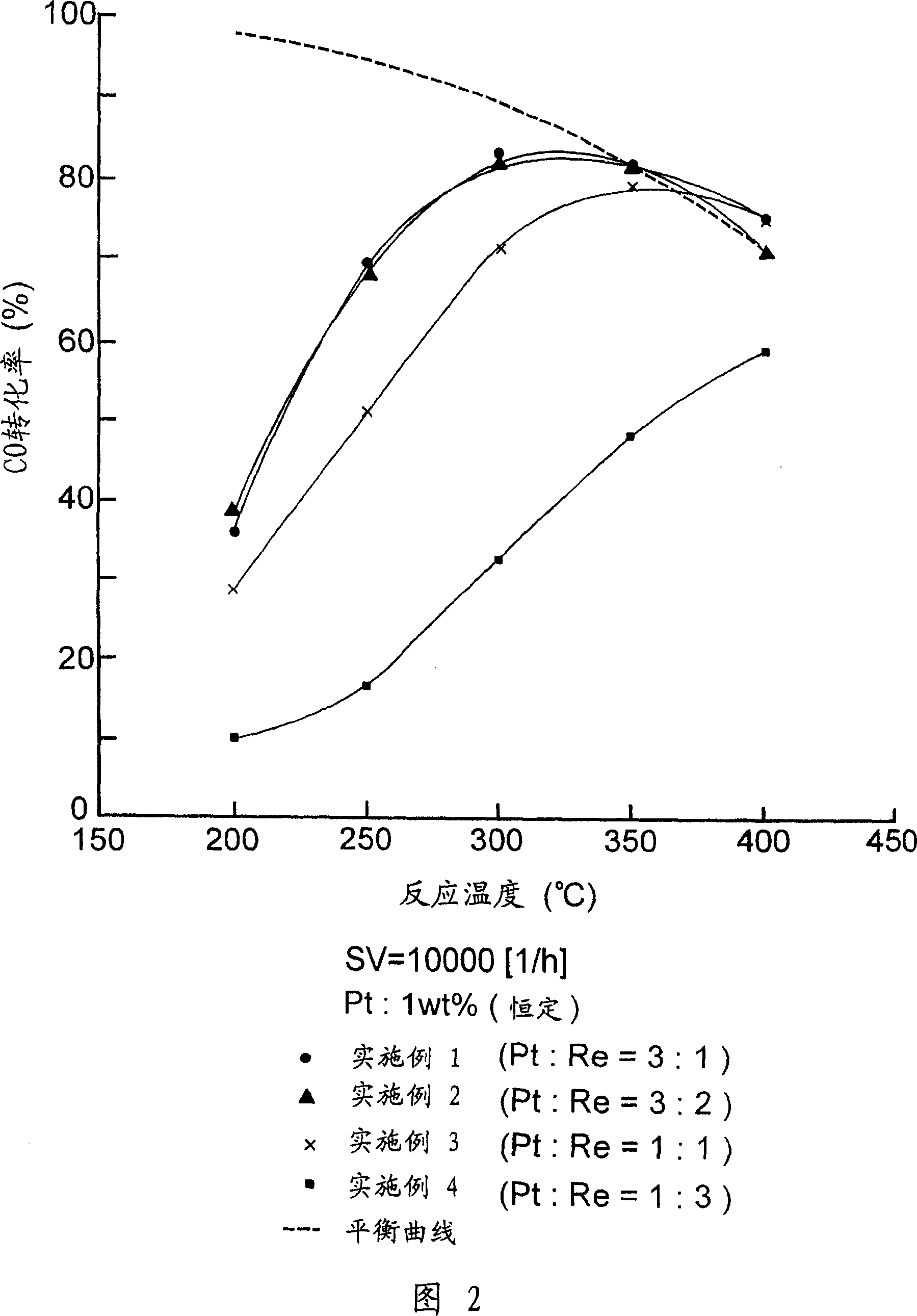
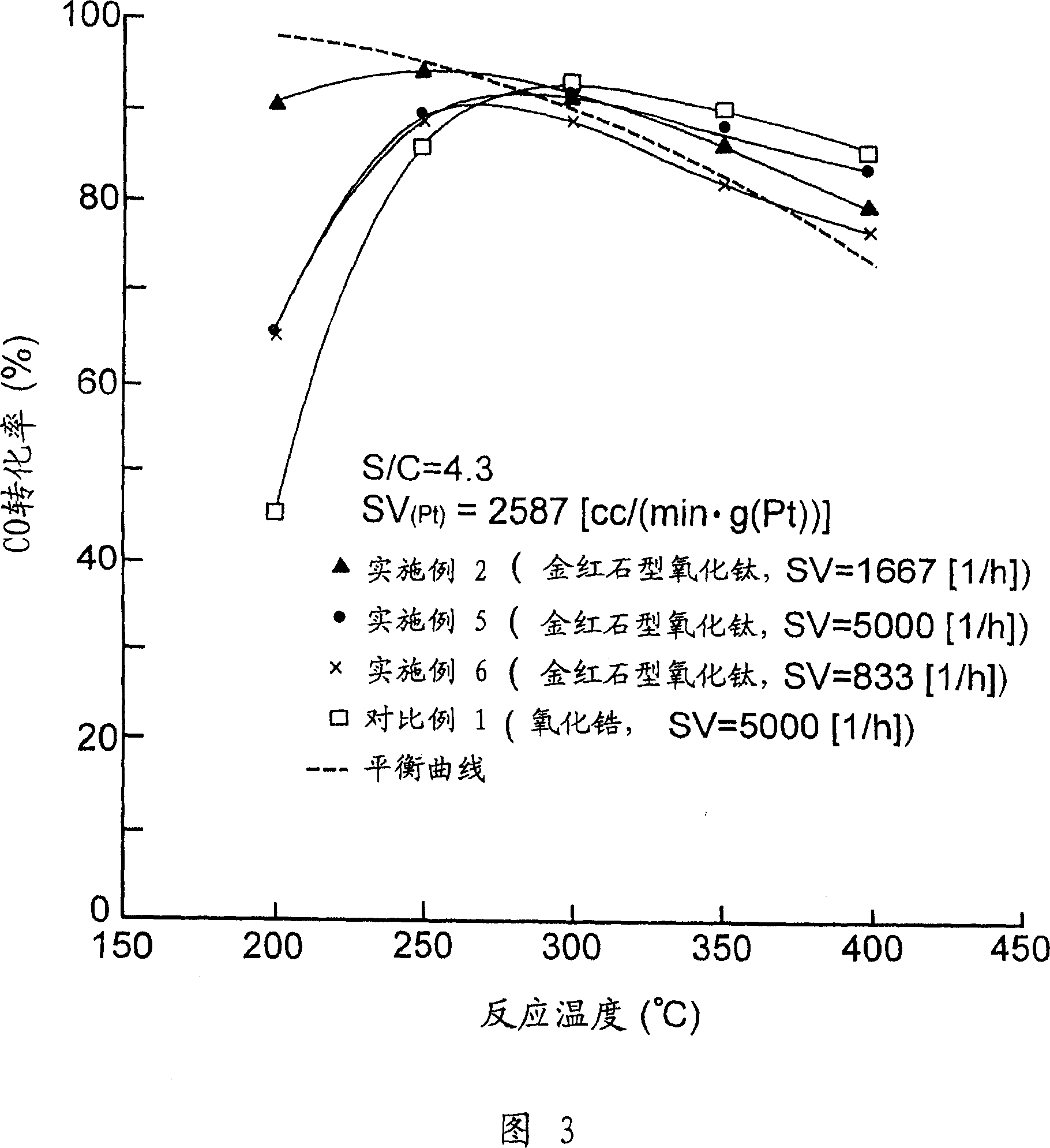
Embodiment 1-6 and comparative example 1
[0041] Using a calciner, rutile titanium dioxide (reference catalyst supplied by Catalysis Society of Japan) was calcined, wherein it was heated to a temperature of 500° C. within 1 hour under an air flow of 60 ml / min, and at this temperature The rutile-type titania carrier of Example 1 was prepared by keeping it at the lower temperature for 1 hour.
[0042] The required amount of the resulting rutile titania support was placed on an evaporating pan located in a hot water bath. Then add pure water to the carrier and mix them intimately. Next, ammonium perrhenate (NH 4 ReO 4 ) (manufactured by NACALAI TESQE INC.) in an aqueous solution was added to the evaporating tray. Pure water was further added to reach a predetermined concentration. By stirring the resulting mixture on the evaporating pan located in the hot water bath, the water trapped in the resulting mixture was evaporated, while the metal salt deposited on the evaporating pan wall was washed with pure water into th...
Embodiment 7
[0061] A required amount of rutile titanium dioxide prepared in the same manner as in Example 1 was placed on an evaporator located in a hot water bath. Then add pure water to the carrier and mix them intimately. Next, a nitric acid solution of dinitrodiamine-platinum(II) (manufactured by TANAKA KIKINZOKU KOGYOK.K.) was added to the evaporating tray. Pure water was further added to reach a predetermined concentration. By stirring the resulting mixture on the evaporating dish placed in a hot water bath, the water trapped in the resulting mixture was evaporated over 2 hours while washing the metal salts deposited on the evaporating dish wall into the bottom of the evaporating dish with pure water. After evaporation, the mixture is further dried at about 100° C. for at least 15 hours so that the platinum is supported on the rutile titanium dioxide.
[0062] Next, the carrier on which the desired amount of platinum was carried was placed on an evaporating tray located in a hot w...
Embodiment 8
[0064] A required amount of rutile titanium dioxide prepared in the same manner as in Example 1 was placed on an evaporator located in a hot water bath. Then add pure water to the carrier and mix them intimately. Next, dinitrodiamine-platinum(II) nitric acid solution (manufactured by TANAKA KIKINZOKU KOGYO K.K.) and ammonium perrhenate (NH 4 ReO 4 ) aqueous solution (manufactured by NACALAI TESQE INC.) was added to the evaporating tray. Pure water was further added to reach a predetermined concentration. By stirring the resulting mixture on the evaporating pan located in a hot water bath, the water trapped in the resulting mixture was evaporated while washing the metal salt deposited on the evaporating pan wall with pure water into the bottom of the evaporating pan. After evaporation, the mixture was further dried at about 100° C. for at least 12 hours in order to simultaneously support platinum and rhenium on the rutile titania. After evaporation, the mixture was dried, c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com