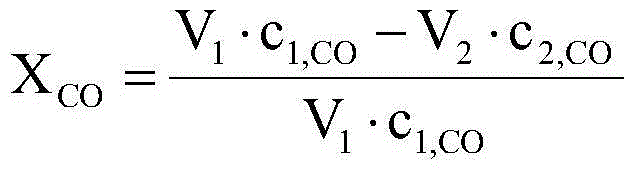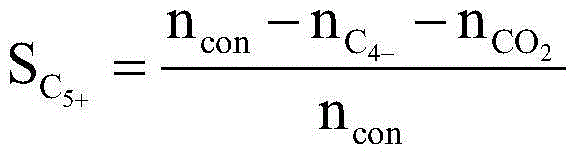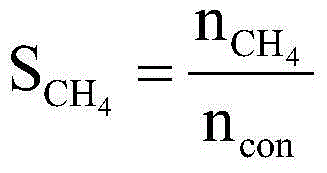Fischer-Tropsch synthesis method
A technology of Fischer-Tropsch synthesis and synthesis gas, which is applied in the preparation of liquid hydrocarbon mixtures and the petroleum industry, and can solve the problems of low CO conversion rate and C5+ selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment is used to illustrate the Fischer-Tropsch synthesis method provided by the present invention.
[0034] (1) Preparation of Fischer-Tropsch synthesis catalyst:
[0035] 10% by weight of β-molecular sieve is mixed with 90% by weight of pseudo-boehmite (Sasol company C1 powder) and extruded with a circular orifice plate with a diameter of 1.4 mm, and then the extruded rod is dried at 120 ° C for 4 hours, and then calcined at 600°C for 4 hours to obtain a carrier. Next, an eggshell catalyst was prepared according to the method disclosed in Example 6 of CN101462079A to obtain a Fischer-Tropsch synthesis catalyst A. The content of the active metal component cobalt in the obtained Fischer-Tropsch synthesis catalyst determined by X-ray fluorescence method was 16% by weight. It can be observed with the naked eye that the active metal component is distributed in an eggshell on the Fischer-Tropsch synthesis catalyst (observed after crushing), and the thickness of ...
Embodiment 2
[0045] This embodiment is used to illustrate the Fischer-Tropsch synthesis method provided by the present invention.
[0046] (1) Preparation of Fischer-Tropsch synthesis catalyst:
[0047]30% by weight of ZSM-22 is mixed with 70% by weight of pseudo-boehmite powder (SB powder purchased from Sasol Company) and then extruded into a circular orifice with a diameter of 1.4 mm, then extruded in Dry at 120°C for 4 hours, and then bake at 600°C for 4 hours to obtain a carrier. Next, an eggshell catalyst was prepared according to the method disclosed in Example 6 of CN101462079A to obtain a Fischer-Tropsch synthesis catalyst B. The content of the active metal component cobalt in the obtained Fischer-Tropsch synthesis catalyst determined by X-ray fluorescence method was 16% by weight. It can be observed with the naked eye that the active metal component is distributed in an eggshell on the Fischer-Tropsch synthesis catalyst (observed after crushing), and the thickness of the shell d...
Embodiment 3
[0051] This embodiment is used to illustrate the Fischer-Tropsch synthesis method provided by the present invention.
[0052] (1) Preparation of Fischer-Tropsch synthesis catalyst:
[0053] 10% by weight of β-molecular sieve is mixed with 90% by weight of pseudo-boehmite (Sasol company C1 powder) and extruded with a circular orifice plate with a diameter of 1.4 mm, and then the extruded rod is dried at 120 ° C for 4 hours, and then calcined at 600°C for 4 hours to obtain a carrier. Next, an eggshell-type catalyst was prepared according to the method disclosed in Example 6 of CN101462079A, and the impregnation solution was replaced with ruthenium nitrate solution of the same concentration and volume to obtain Fischer-Tropsch synthesis catalyst C. The content of the active metal component ruthenium in the obtained Fischer-Tropsch synthesis catalyst determined by X-ray fluorescence method was 2% by weight. It can be observed with the naked eye that the active metal component is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com