Mannosan peptide injection and its preparation and use method
A mannanpeptide and injection technology, which is applied in the field of mannanpeptide injection and mannanpeptide injection preparation, can solve the problems of medical accidents, prone to infusion reactions, secondary pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
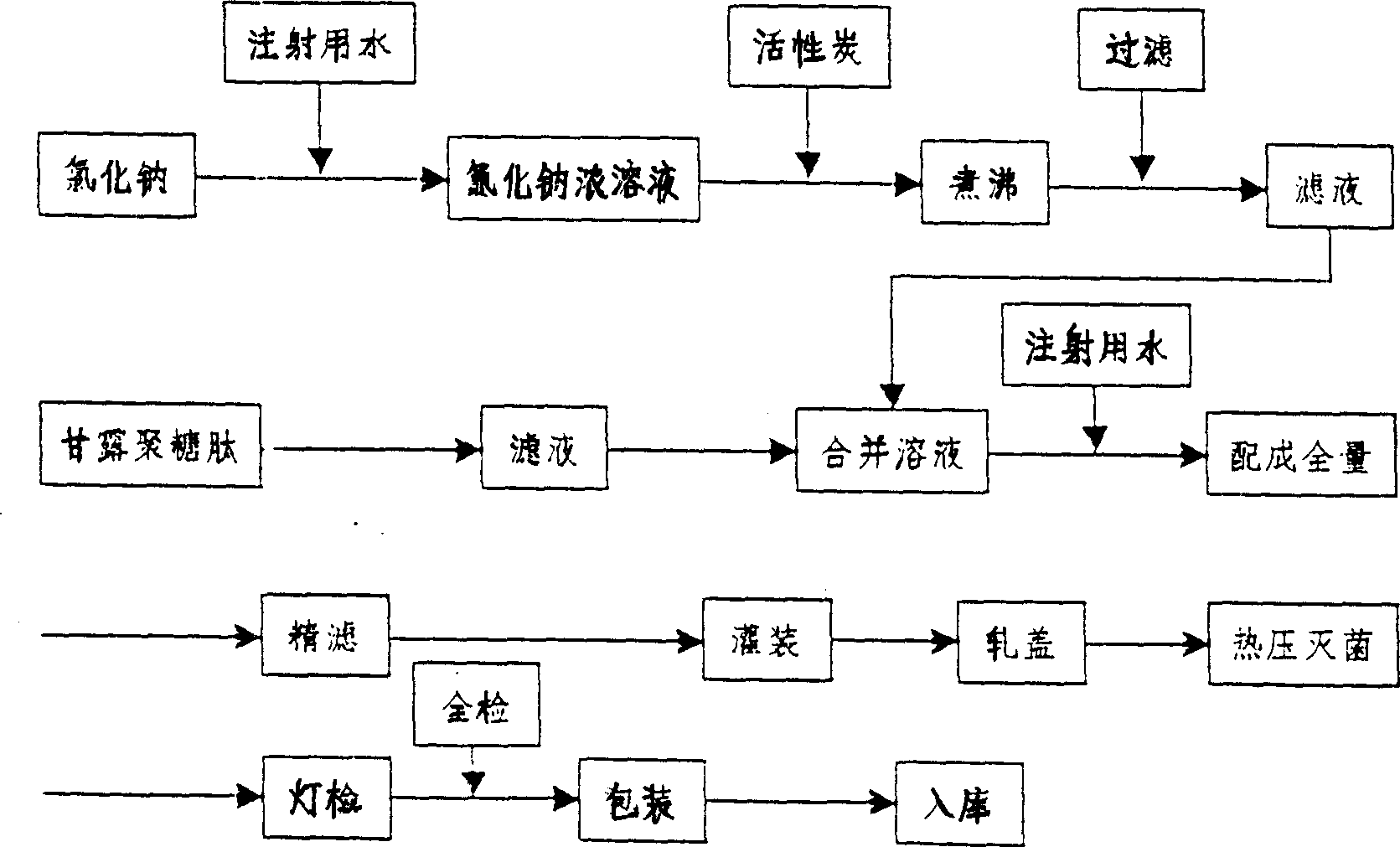
[0056] Mannan peptide injection with a specification of 100 ml was prepared, which contained 10 mg of mannan peptide and 0.9 g of sodium chloride.
[0057]The formula or prescription is:
[0058] Mannan peptide (according to dry product) 0.1g
[0059] Sodium chloride 9.0g
[0060] Add water for injection to 1000ml
[0061] Among them, mannan peptide is the main drug of this product, and sodium chloride is the isotonic regulator.
[0062] Process method:
[0063] Take the prescribed amount of sodium chloride for injection and an appropriate amount of water for injection to prepare a concentrated solution of about 20% sodium chloride, add an appropriate amount of activated carbon, stir evenly, heat to a boil of about 15 mm, filter, and the filtrate is set aside.
[0064] Take the prescribed amount of mannan peptide and add an appropriate amount of water for injection to dissolve, filter to obtain a filtrate.
[0065] Combine the above two solutions and add water...
Embodiment 2
[0076] Mannan peptide injection with a specification of 100 ml was prepared, which contained 10 mg of mannan peptide and 5 g of glucose.
[0077] The formula or prescription is:
[0078] Mannan peptide (according to dry product) 0.1g
[0079] Glucose 50g
[0080] Add water for injection to 1000ml
[0081] Among them, mannan peptide is the main drug of this product, and glucose is the isotonic regulator.
[0082] Process method:
[0083] Take the prescribed amount of glucose and an appropriate amount of water for injection to prepare a concentrated glucose solution of about 20%, add an appropriate amount of activated carbon, stir evenly, heat to a boil of about 15mm, filter, and the filtrate is set aside.
[0084] Take the prescribed amount of mannan peptide and add water for injection to the prescribed amount, filter to obtain the filtrate.
[0085] Combine the above two solutions and add water for injection to the prescribed amount, filter through a 0.22um mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap