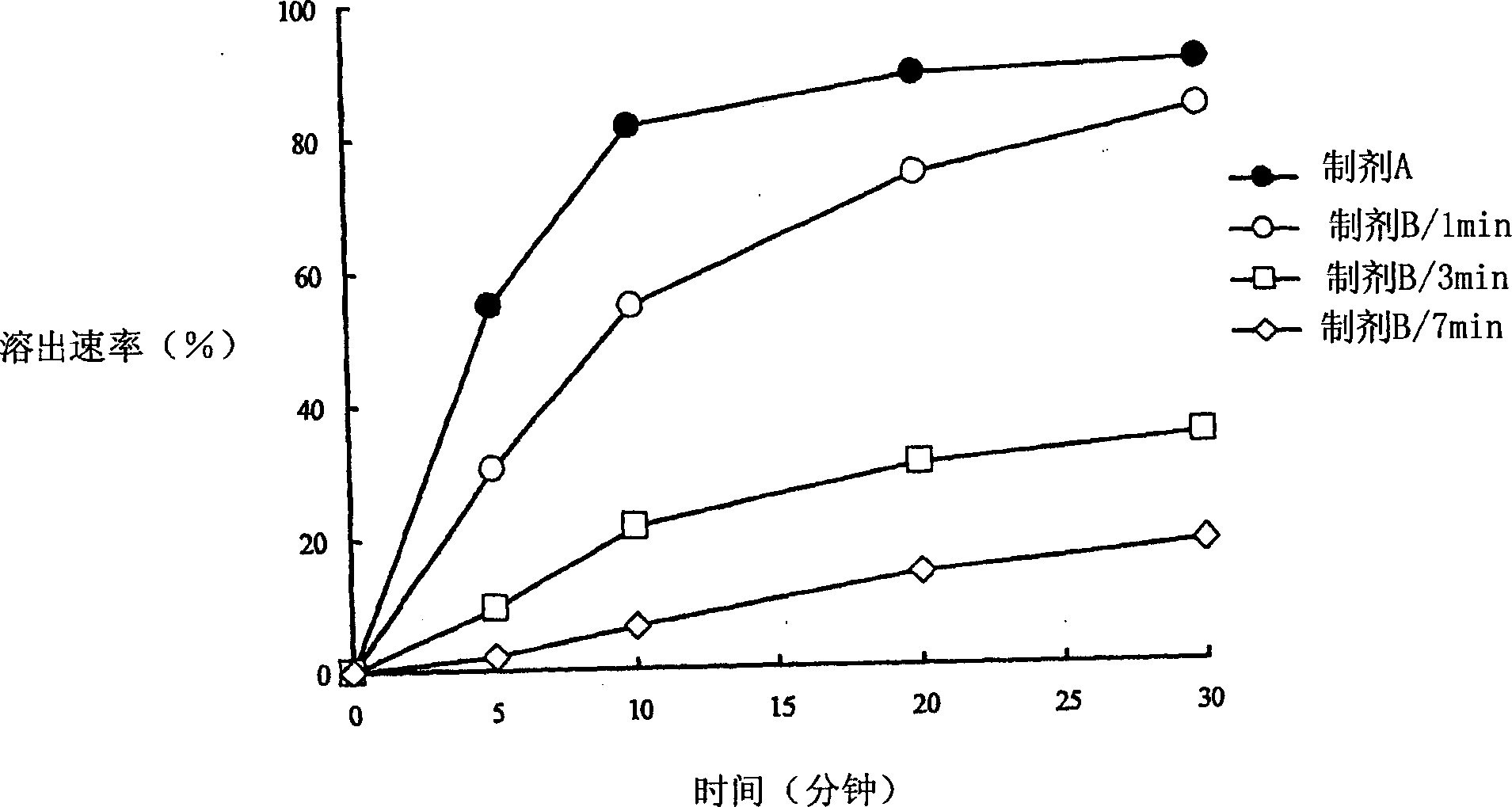
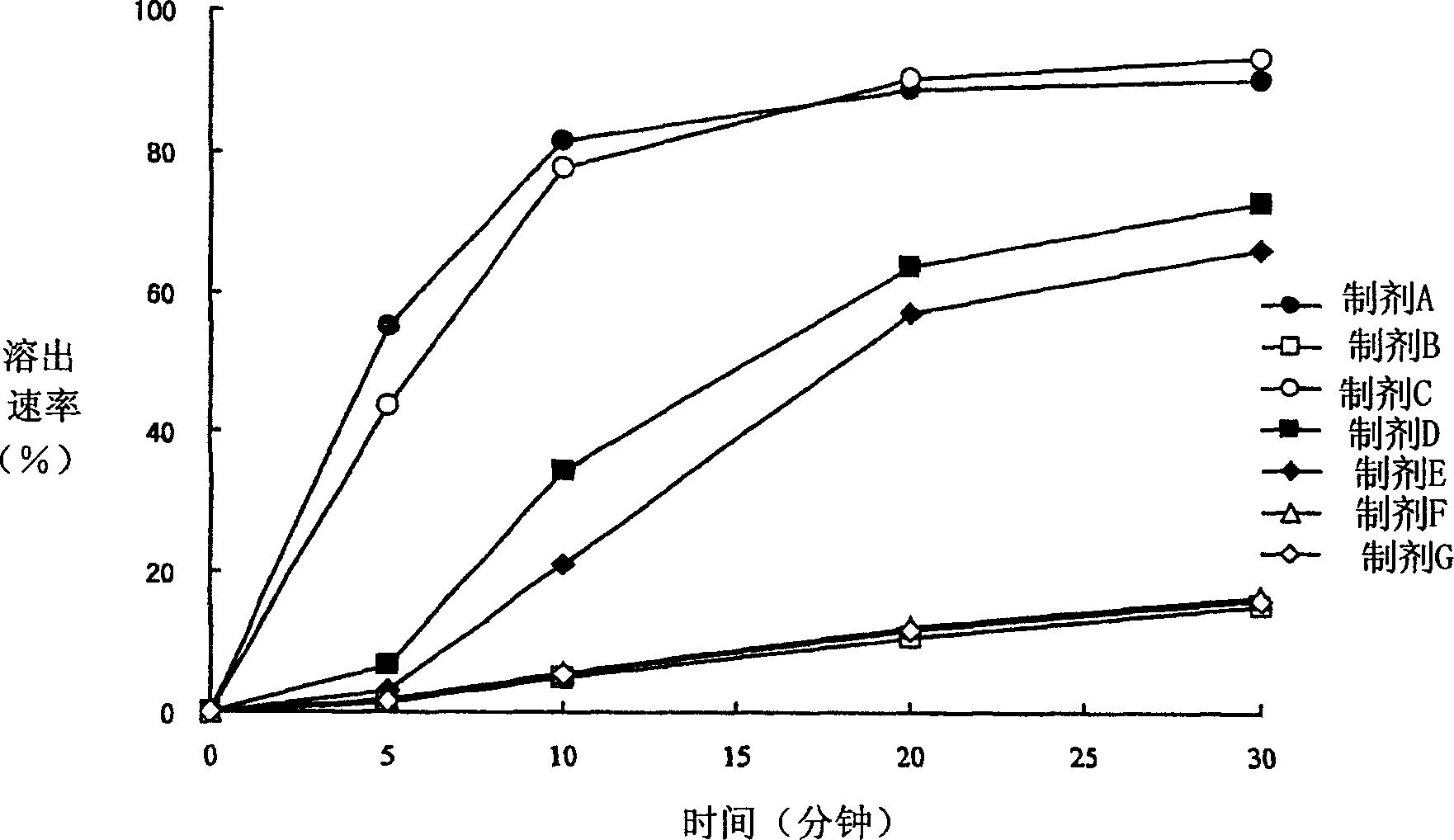
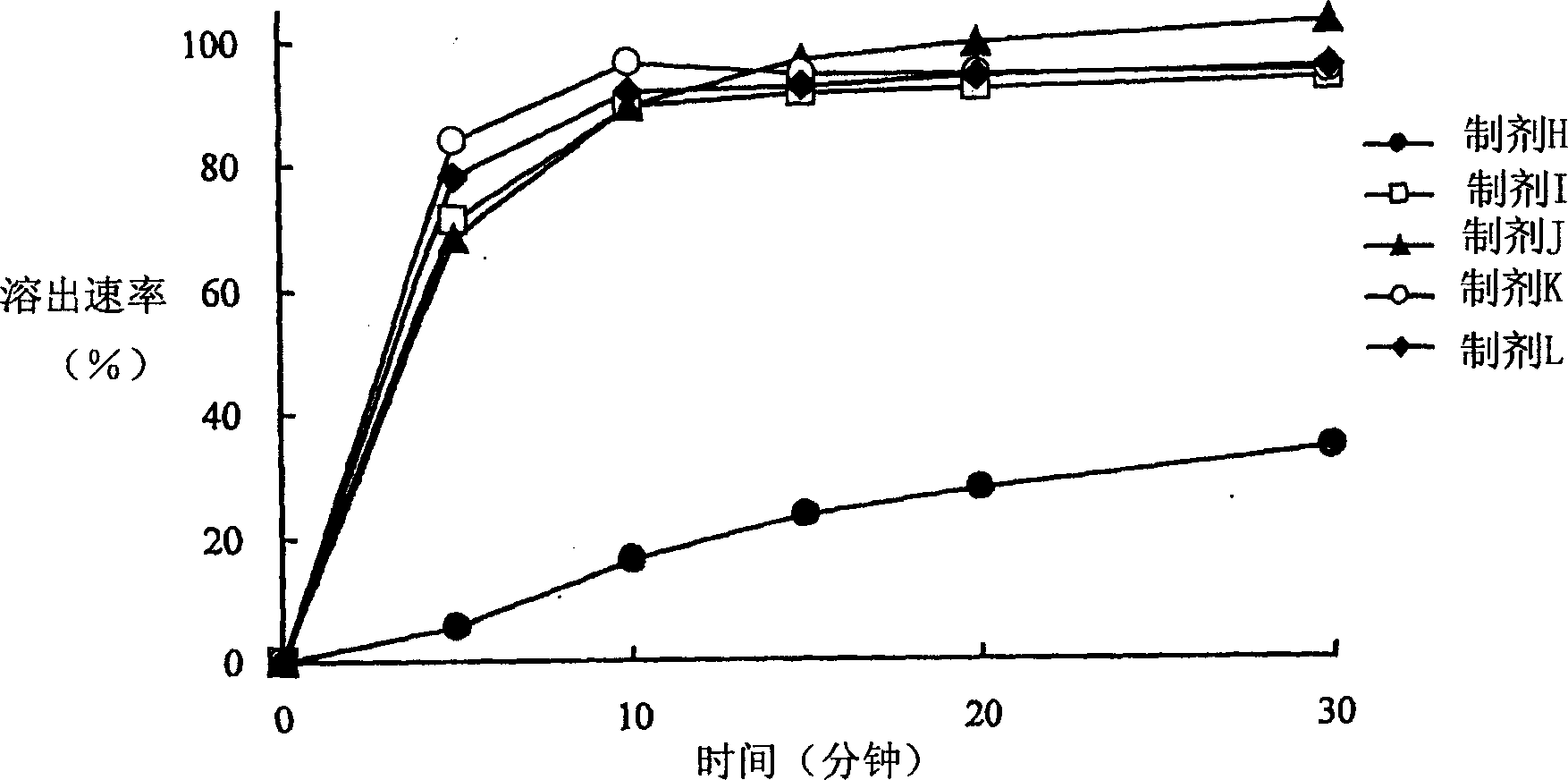
Solid drug for oral use
一种药物、剂型的技术,应用在口服形式的固体药物领域,能够解决没有教导或提示等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Capsules containing 2.0 mg KMD-3213
[0130] Thoroughly mix 2.0 parts of KMD-3213, 134.4 parts of D-mannitol, 26.0 parts of partially pregelatinized starch (PCS (registered trademark), Asahi Chemical Industry Co., Ltd) and 9.0 parts of partially pregelatinized starch (Starch 1500 (registered trademark) trademark), Japan Colorcon Co., Ltd). An appropriate amount of water is added, and the mixture is granulated. The particles were dried with a fluidized bed dryer at an inlet temperature of 60°C until the exhaust temperature reached 40°C, and then sieved. A mixture of 1.8 parts of magnesium stearate and 1.8 parts of sodium lauryl sulfate was added to the sieved granules, mixed for 5 minutes, and the mixture was filled into capsule shells to prepare capsules containing 2.0 mg of KMD-3213.
Embodiment 2
[0132] Capsules containing 4.0 mg KMD-3213
[0133] Thoroughly mix 4.0 parts of KMD-3213, 132.4 parts of D-mannitol, 26.0 parts of partially pregelatinized starch (PCS (registered trademark), Asahi Chemical Industry Co., Ltd) and 9.0 parts of partially pregelatinized starch (Starch 1500 (registered trademark) trademark), Japan Colorcon Co., Ltd). An appropriate amount of water is added, and the mixture is granulated. The particles were dried with a fluidized bed dryer at an inlet temperature of 60°C until the exhaust temperature reached 40°C, and then sieved. A mixture of 1.8 parts of magnesium stearate and 1.8 parts of sodium lauryl sulfate was added to the sieved granules, mixed for 5 minutes, and the mixture was filled into capsule shells to prepare capsules containing 4.0 mg of KMD-3213.
Embodiment 3
[0135] Tablets containing 4.0 mg KMD-3213
[0136] 4.0 parts of KMD-3213, 117.0 parts of D-mannitol, and 7.0 parts of low-substituted hydroxypropylcellulose (L-HPC (registered trademark), Shin-Etsu Chemical Co., Ltd.) were thoroughly mixed. A 12% aqueous solution of hydroxypropylcellulose (4 parts of hydroxypropylcellulose and about 30 parts of water) was added and the mixture was granulated. Dry the particles with a fluidized bed dryer at an inlet temperature of 60°C until the exhaust temperature reaches 40°C, dry and sieve and pass through a sieve. Add 1.0 parts magnesium stearate to the granules and mix for 3 minutes. The mixture was compressed into tablets and coated with a coating agent to prepare tablets containing 4.0 mg of KMD-3213.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com