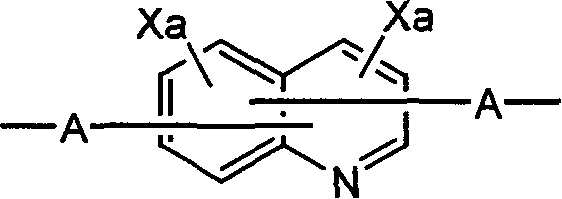
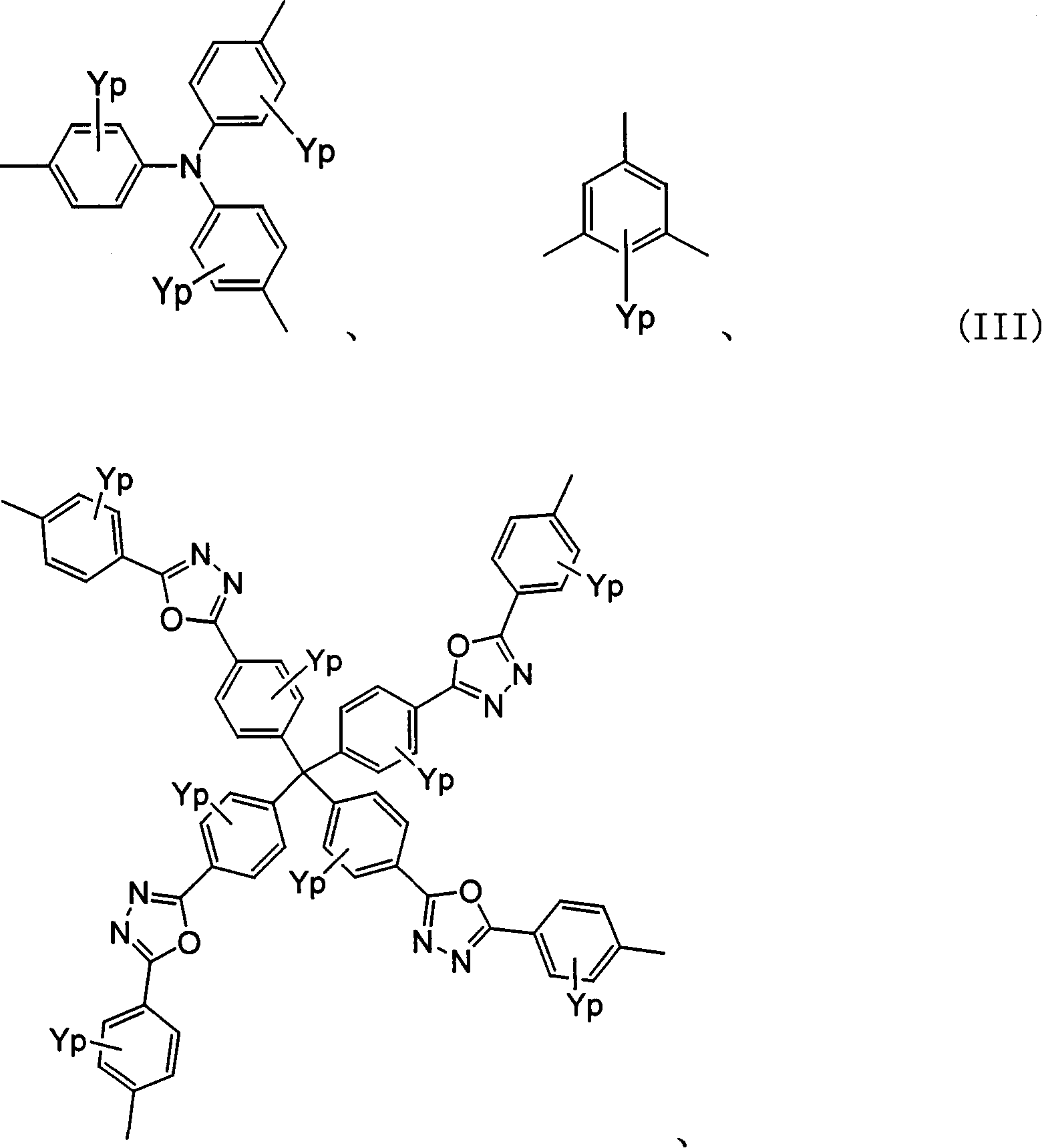
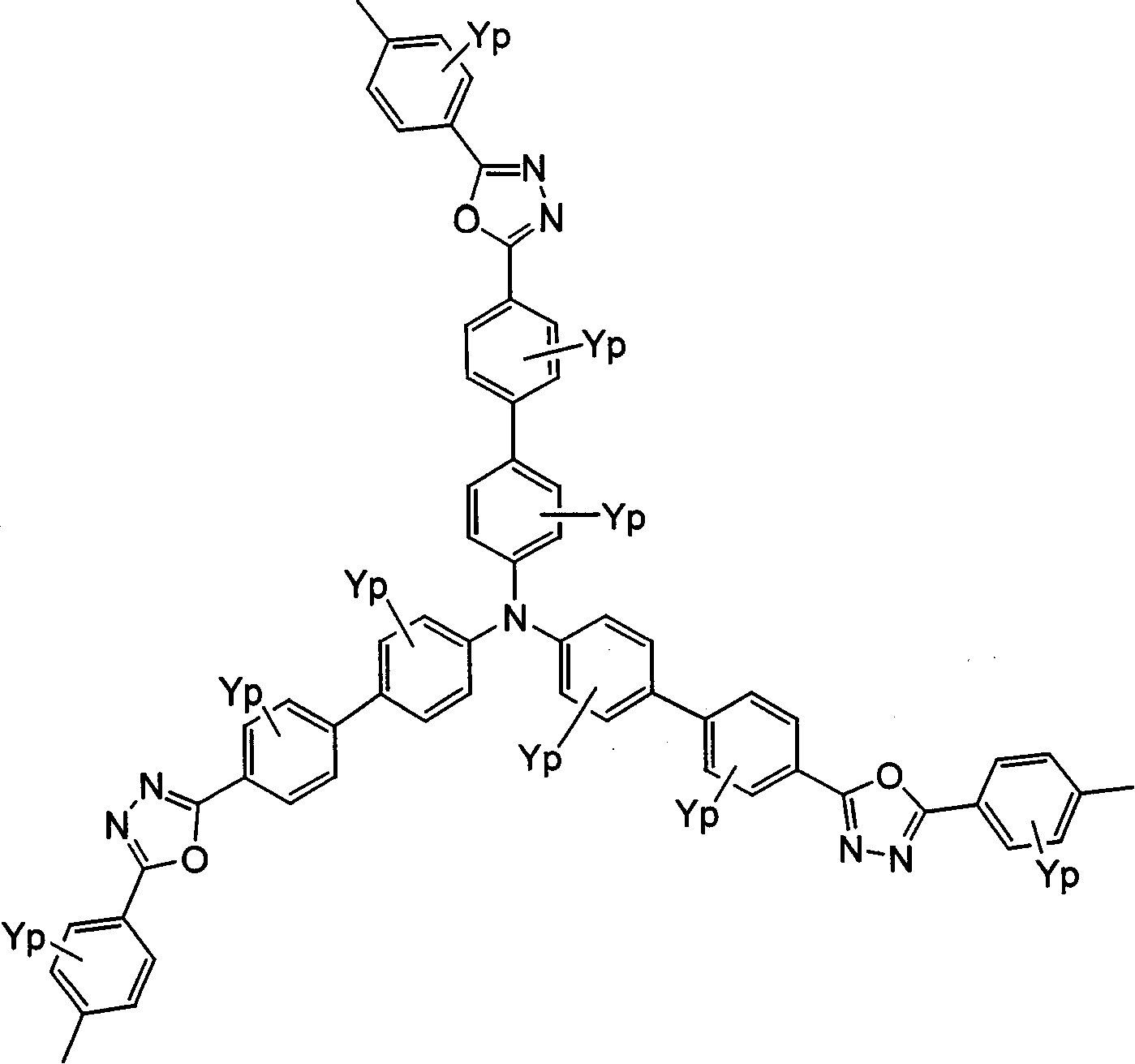
Polyquinoline copolymer having a branched structure and organic electroluminescent device using same
A technology of branched chain structure and polyquinoline, which is applied in electroluminescent light sources, electrical components, organic semiconductor devices, etc., and can solve problems such as short luminous life and undiscovered products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] The synthesis of embodiment 1 quinoline derivative diboronic acid ester
[0121] 6,6'-bis[2-(4-bromophenyl)-3,4-diphenylquinoline](6,6'-bis[2-(4-bromophenyl)-3,4-diphenylquinoline] ) (30mmol) in tetrahydrofuran (THF, Tetrahydrofuran) solution, under argon flow, while stirring carefully while slowly adding magnesium (1.9 grams (g), 80 millimoles (mmol)) in THF mixture, to prepare grid Grignard reagent. The obtained Grignard reagent was slowly dropped into a THF solution of trimethylboric acid ester (300 mmol) at -78° C. while carefully stirring for 2 hours, and then, at room temperature Stir for 2 days. The reaction mixture was pulverized, poured into ice-containing 5% dilute sulfuric acid, and stirred. The obtained aqueous solution was extracted with toluene, and the extract was concentrated to obtain a colorless solid. The obtained solid was recrystallized from toluene / acetone (1 / 2) to obtain colorless crystals of quinoline derivative diboronic acid (40%). The obt...
Embodiment 2
[0122] Synthesis (1) of the copolymer of embodiment 2 quinoline derivatives and branched chain structure derivatives
[0123] In the tribromo branched chain structure monomer (1mmol) represented by the following structural formula, the dialkoxy dibromobenzene compound (9mmol) represented by the following structural formula, the quinoline derivative diboron synthesized by the method of Example 1 Ester (10mmol) and Pd(O)(PPh 3 ) 4 (0.2mmol) of toluene solution, under argon flow, add 2M potassium carbonate (K 2 CO 3 ) solution, while vigorously stirring, while carrying out reflux for 48 hours.
[0124]
[0125]
[0126] After cooling the reaction mixture to room temperature, it was poured into a large amount of methanol to precipitate a solid. The precipitated solid was suction-filtered and washed with methanol to obtain a solid. The filtrated solid was dissolved in toluene, and poured into a large amount of acetone to precipitate the solid. The precipitated solid was...
Embodiment 3
[0127] Synthesis (2) of the copolymer of embodiment 3 quinoline derivatives and branched chain structure derivatives
[0128]6,6'-bis[2-(4-fluorophenyl)-3,4-diphenylquinoline](6,6'-bis[2-(4-fluorophenyl)-3,4-diphenylquinoline]) (9mmol), the branched chain structural monomer (1mmol) represented by the following structural formula, the dialkoxy dihydroxybenzene compound (9mmol) represented by the following structural formula, potassium carbonate (15mmol), anhydrous NMP (40 milliliters (ml) ) and anhydrous toluene (20ml), under a nitrogen stream, while vigorously stirring, heated and refluxed for 30 hours.
[0129]
[0130] After adding NMP (60 ml) to the reaction mixture, it was cooled to room temperature. The resulting solution was poured into a large amount of distilled water to precipitate a solid. The precipitated solid was suction-filtered and washed with distilled water, methanol, and acetone to obtain a solid. The filtrated solid was dissolved in toluene, and poured...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com