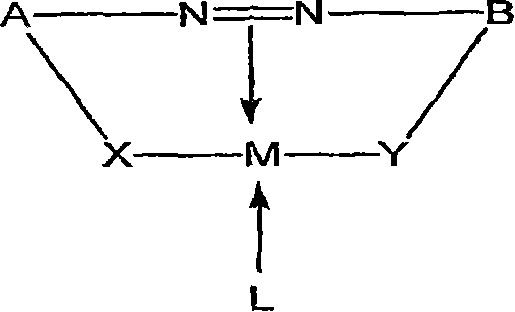
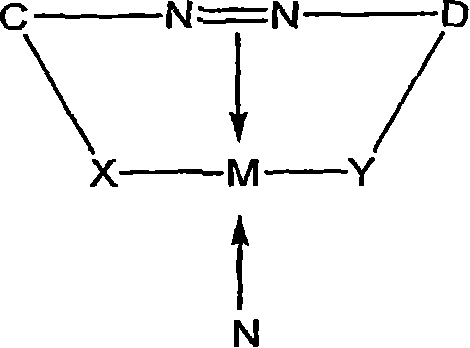
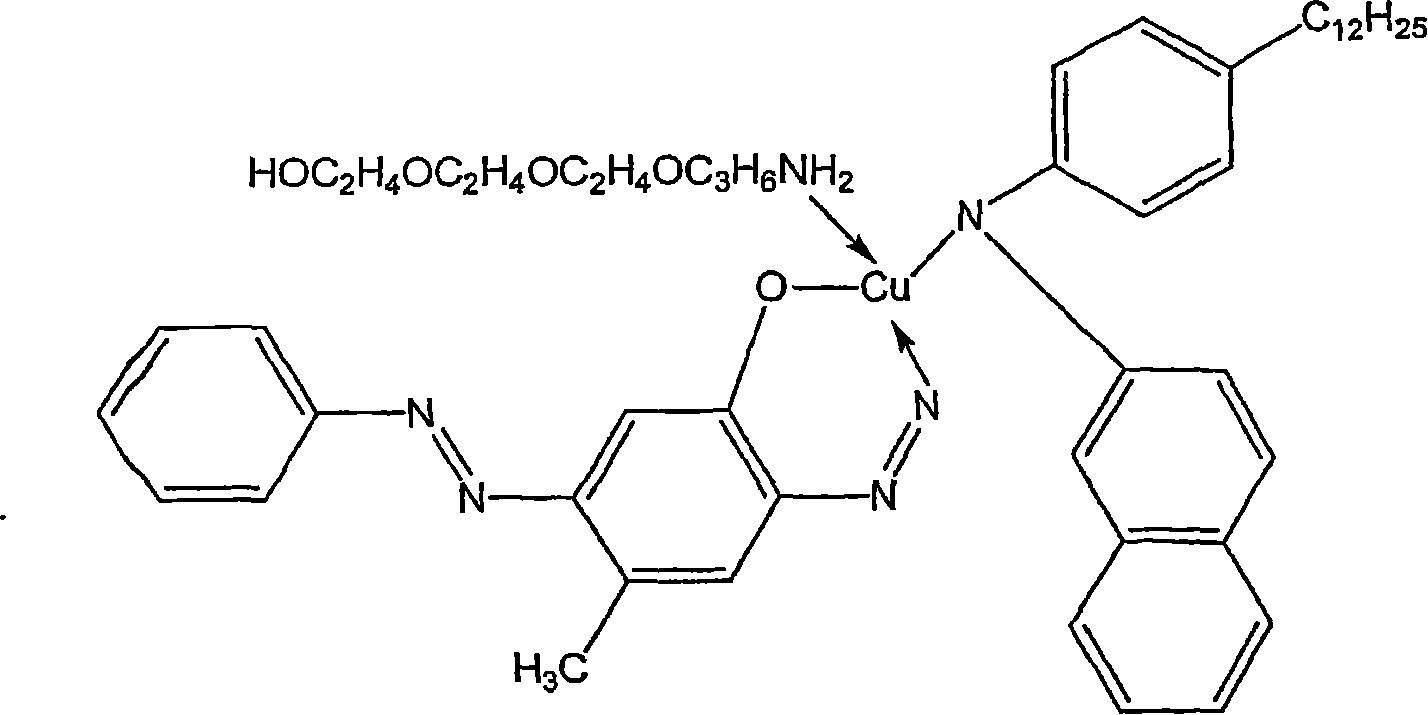
Organic solvent soluble metal complex azo dyes
一种有机溶剂、染料的技术,应用在偶氮染料、有机染料、偶氮染料的配合金属化合物等方向,能够解决没有溶解性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]To a solution of 15 g of sodium nitrite in 60 ml of water was added 28 g of technical grade (0.2 mole) 4-nitroaniline and stirred until a homogeneous slurry was obtained. The slurry was then added over about 5 minutes to 60 g of 32% hydrochloric acid which had been cooled to -5°C by adding ice. Additional ice was provided to the ligand system to prevent the temperature from rising above 5°C during slurry addition. The mixture is then stirred for about 15 minutes until it becomes as clear as possible. At this point, a small amount of filter aid was added to filter 4-nitrobenzenediazonium chloride while still keeping its temperature below 5°C. The filtered solution is then transferred to a stirred beaker or flask and any remaining nitrite ions are reduced with sulfamic acid. Next, 32 g of 2,5-dimethoxyaniline dissolved in 200 ml of water and 25 g of 32% hydrochloric acid were added over 15 minutes via the dropping funnel. The azo coupling started immediately and a visco...
Embodiment 2
[0041] Into a 5 liter flask surrounded by an ice bath was added 300 g of ice and 50 g of water. The mixture was stirred and 130 g of 96% sulfuric acid was added, followed by 100 g of aniline, forming a white dispersion of aniline sulfate. The mixture was then cooled to -5°C by adding ice and diazotized below 3°C by adding 190 g of 40% aqueous sodium nitrite solution. The mixture was stirred until all white particles of aniline sulfate had dissolved to form a clear solution. After approximately 5 minutes of agitation, unreacted nitrite ions were removed by adding a small amount of sulfamic acid. 140 g of 2-methoxy-5-methylaniline was added to the diazo compound while raising the temperature to 5-10°C. After stirring for 15 minutes, sufficient 30% aqueous sodium acetate was added to raise the pH of the reaction to 3.5. The reaction was stirred at 10-12°C for 4 hours to substantially complete the azo coupling. Next, 600 g of xylene was added, followed by 110 g of concentrated...
Embodiment 3
[0044] The synthesis of Example 2 was repeated except that 165 g of diethylene glycol-3-aminopropyl ether was replaced by 220 g of 1-propylamine-3,3'-[oxybis(2,1-ethanedyloxy)]bis. The resulting dye had very similar properties to Example 2, except that it was significantly bluer in the shade.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com