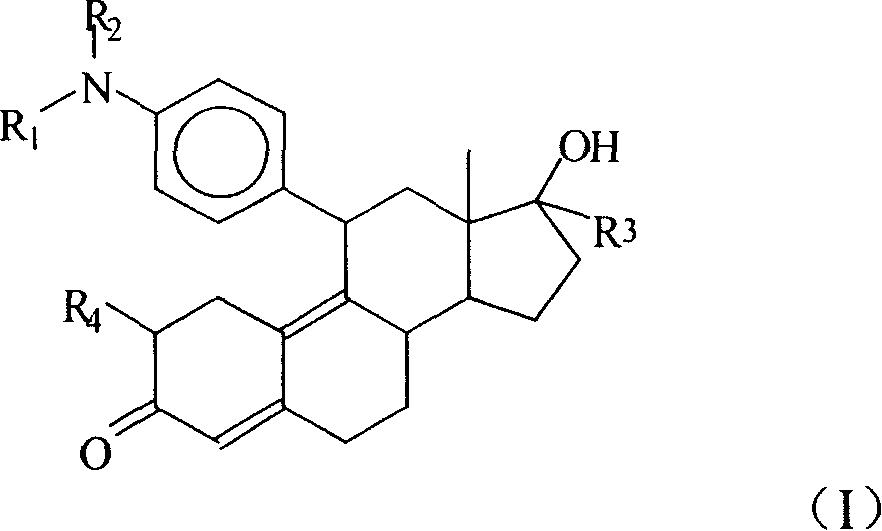
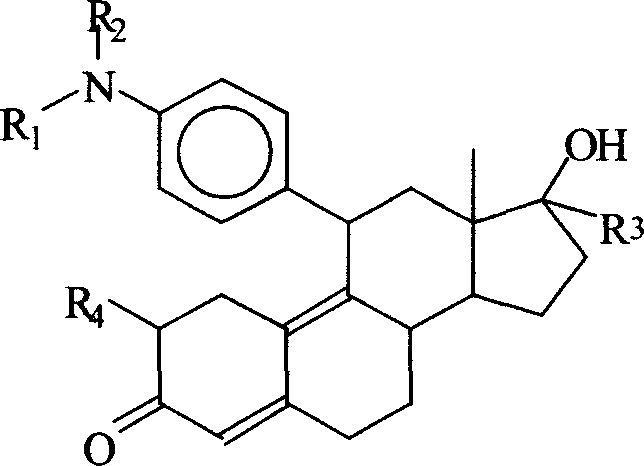
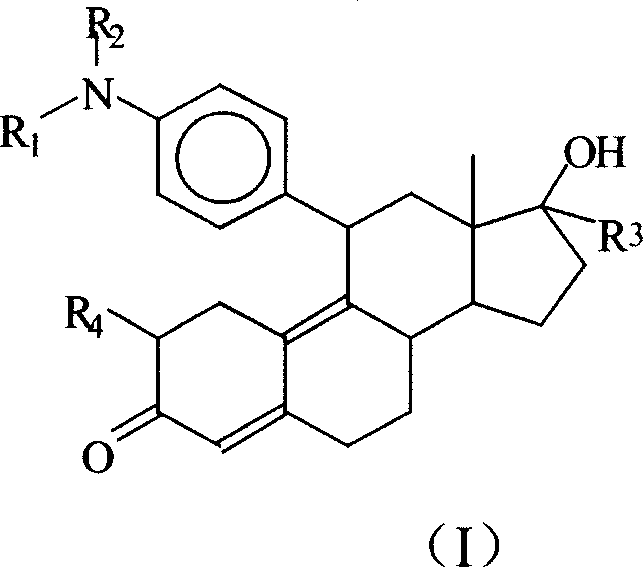
Use of symipristone compound for treating AIDS
A compound and AIDS technology, which is applied in the field of preparation of AIDS drugs, cymipristone compounds, can solve the problems of delayed drug resistance, emergence, and inability to fundamentally solve the problem of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation method of Cymipristone
[0031] Synthetic route of cymipristone:
[0032]
[0033] Epoxy adduct Cymipristone
[0034] (1) Preparation of 4-(N-methyl-N-cyclohexylamino)phenylmagnesium bromide
[0035] In a four-neck flask, put 1.4 grams of magnesium flakes (Mg) and 10 milliliters of anhydrous tetrahydrofuran (THF), without adding iodine or a little iodine, and drop 10.86 grams of 4-bromo-N-methyl- N-cyclohexylaniline (dissolved in 24 milliliters of anhydrous tetrahydrofuran), dropwise, continue to insulate and stir for 1 hour to obtain a tetrahydrofuran solution of 4-(N-methyl-N-cyclohexylamino)phenylmagnesium bromide (to be step addition reaction).
[0036] (2) 3,3-ethylenedioxy-5α, 17β-dihydroxy-11β-[4-(N-methyl-N-cyclohexylamino)phenyl]-17α-(1-propynyl)- Preparation of 9(10)-estrene (adduct)
[0037] In a four-neck bottle, put 5 grams of 3,3-ethylenedioxy-5,10-epoxy-17α-(1-propynyl)-17β-hydroxyl-9(11)-estrene (epoxy matter), 29.1...
Embodiment 2
[0044] Embodiment 2: Determination of human-derived glucocorticoid receptor binding activity
[0045] Purpose of the test: To detect the binding ability of cymipristone to the human glucocorticoid receptor and compare it with Mifepristone which has a similar chemical structure.
[0046] (1) Test method
[0047] The reaction buffer consists of 25mM NaPO 4 , 20mM NaMoO 4 , 10mM KF and 10% Glycerol (pH=7.3), stored at 4°C until use. The stock solution was prepared from CHAPS and DTT and stored at -20°C for future use. Positive drug (Dexamethasone) and test compound solutions were prepared according to a certain concentration gradient, and 2.5 μl was added to each well of a 96-well plate. Take out the required amount of reaction buffer and add it to the stock solution to make the final concentration reach CHAPS 0.25mM and DTT 2mM. Add protease inhibitors (Aprotinin and Leupeptin) to a final concentration of 1 μg / μl and place in an ice bath. After human nuclear receptors GR (...
Embodiment 3
[0052] Example 3: Detection of the binding activity of cymipristone to rat liver cell plasma glucocorticoid receptors Ketone (Mifepristone) for comparison.
[0053] (1) Test method
[0054] Female clean SD rats, weighing 200-250 grams, were fed quantitatively and had free access to water. The breeding temperature is controlled at about 24 degrees Celsius, and the light is 11 hours during the day-13 hours at night. All experimental animals were observed for 1 week before the experiment.
[0055] Bilateral incisions were made under the dorsal rib arch of the rats, and the bilateral adrenal glands of the rats were removed. After the operation, they were fed with normal saline to maintain water and electrolyte balance.
[0056] On the third day after the operation, the animals were sacrificed acutely, and the liver was quickly taken out and placed in ice-cold buffer solution, and the blood was washed as much as possible. Take a certain amount of tissue and add buffer at a rati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com