Miniaturized antibodies of antiglucocorticoids induced tumor necrosis factor receptor (GITR), and polymers and application thereof
A technology of tumor necrosis factor and corticosteroids, applied in the direction of anti-tumor drugs, antibody mimics/stents, applications, etc., can solve the problems of limited application range, complicated preparation process, poor tissue permeability, etc., and achieve anti-immune diseases/disorders Proliferative diseases/disorders, simple screening process, good tissue penetration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0287] In another aspect, the present invention provides a method comprising preparing the antibody of the present invention or its antigen-binding fragment, the fusion protein of the present invention or the immunoconjugate of the present invention (referred to as the preparation method of the present invention or the method of the present invention), Including: culturing the host cell of the present invention under conditions suitable for expressing the antibody or antigen-binding fragment thereof, fusion protein or immunoconjugate of the present invention.
[0288] In some embodiments, the methods of the present invention also include the use of any expression means that facilitate the expression and purification of the antibodies or antigen-binding fragments thereof, fusion proteins or immunoconjugates of the present invention, such as the use of secretion signal sequences, expression enhancing elements , high-efficiency promoters, and any other expression and regulatory el...
Embodiment 1
[0316] Example 1. Screening of anti-GITR Nanobodies
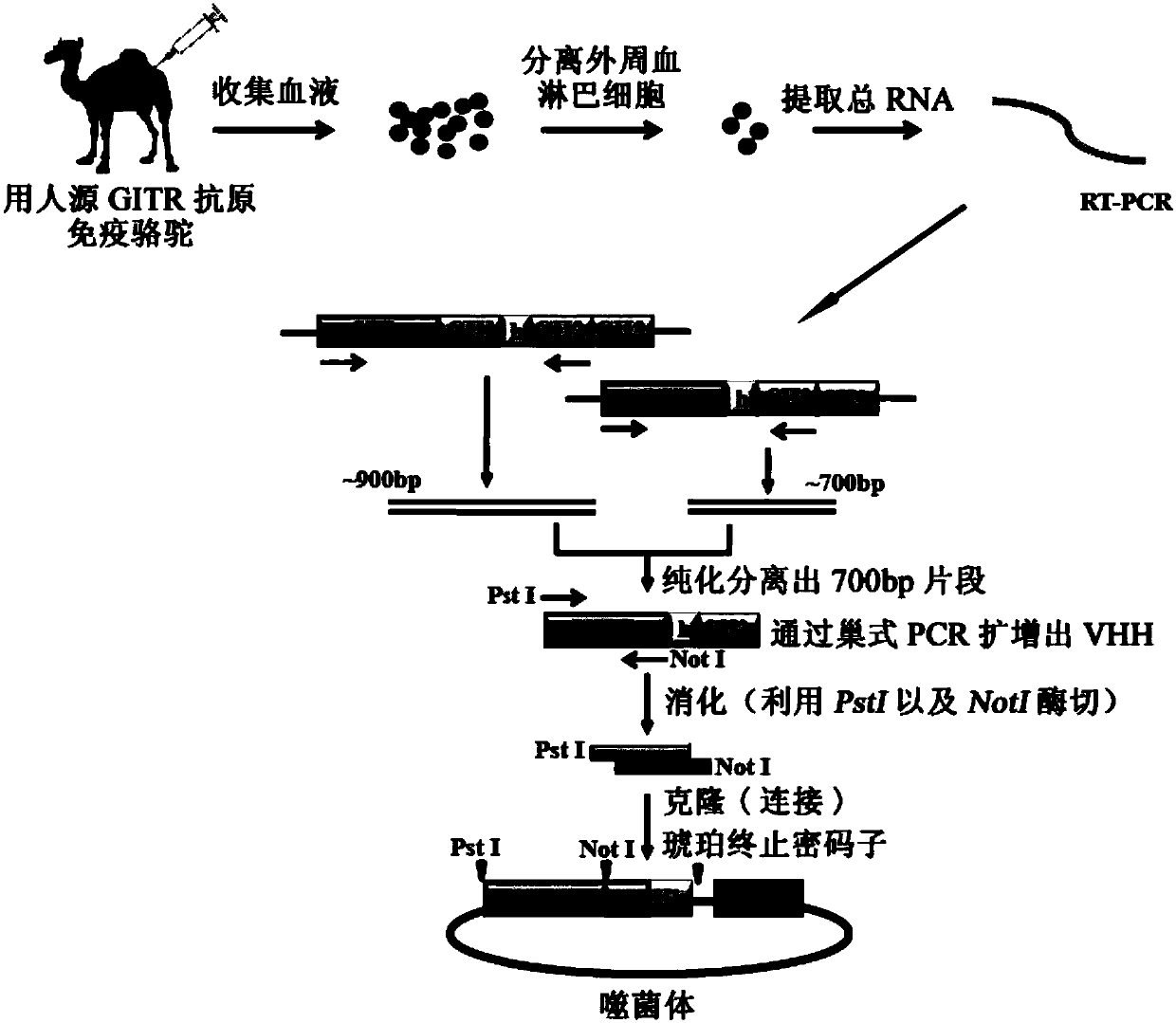
[0317] 1. Camel immunity
[0318] 1 mg of antigen GITR-His (Acro biosystems) was mixed with Freund's adjuvant (Sigma) in equal volume and divided into two tubes to immunize two Xinjiang Bactrian camels. In this way, immunization is performed once a week, and a total of 7 times are immunized to stimulate B lymphocytes to express antigen-specific nanobodies. After seven times of immunization, the serum titer of GITR-His Nanobody produced in Bactrian camels in Xinjiang can reach more than 1:1000, thus confirming that the desired Nanobody is produced in camels.
[0319] 2. Construction of phage display nanobody library
[0320] (1) After 7 times of immunization, extract 100ml camel blood, separate peripheral blood lymphocytes, and extract total RNA;
[0321] (2) Utilize RT-PCR to reverse transcribe the total RNA obtained in step (1) into cDNA, then utilize nested PCR (twice PCR) to amplify to obtain VHH, and its amplificatio...
Embodiment 2
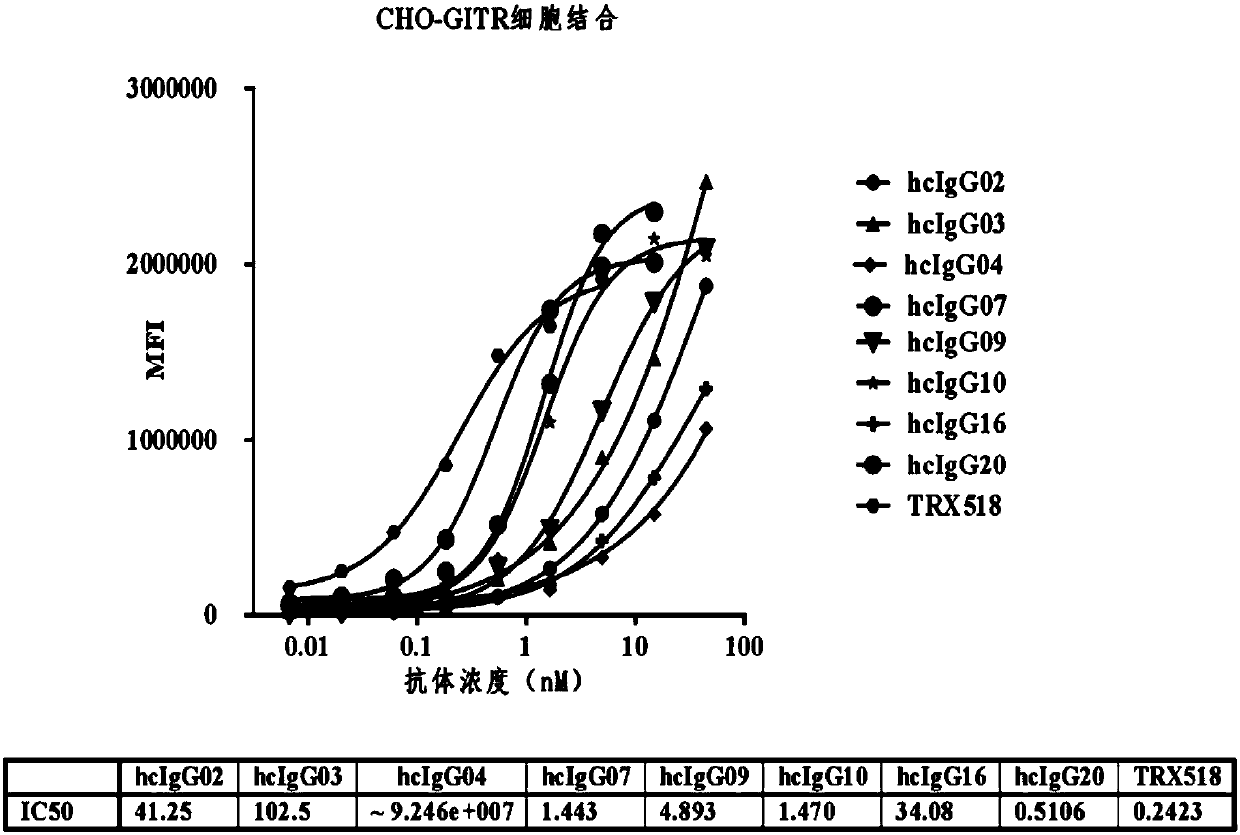
[0342] Example 2. Construction, expression and purification of heavy chain antibodies
[0343] 1. Construction of heavy chain antibody (hcIgG):
[0344] (1) PCR amplification of cDNA of Nanobodies Nb-02, Nb-03, Nb-04, Nb-07, Nb-09, Nb-10, Nb-16, Nb-20.
[0345] (2) The PCR fragments obtained from gel recovery were constructed into the HEK293 expression vector pTT5 (Biotechnology Research Institute; Montreal, Canada) by homologous recombination (Vazyme, C112-01 / 02), which contained the following DNA sequences: IgG1Fc fragment:
[0346] GACAAAACCCACACCTGTCCCCCTTGTCCTGCTCCCGAGCTCCTGGGAGGACCTTCCGTGTTCCTCTTCCCTCCCAAACCCAAGGACACCCTGATGATTAGCAGGACACCCGAGGTGACCTGTGTGGTGGTGGATGTGAGCCATGAGGACCCCGAGGTGAAGTTTAACTGGTACGTGGACGGCGTCGAGGTGCACAACGCTAAGACCAAACCCAGGGAGGAGCAGTACAACTCCACATACCGGGTCGTGAGCGTGCTGACCGTCCTGCACCAGGATTGGCTGAATGGCAAGGAGTACAAGTGCAAGGTGAGCAACAAGGCCCTGCCCGCCCCCATCGAGAAGACCATCAGCAAGGCCAAAGGACAGCCTCGGGAGCCCCAGGTTTATACTCTCCCCCCCAGCCGGGACGAACTGACCAAGAATCAGGTGTCCCTCACCTGCCTCGTGAA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com