Stabilized cholinesterase substrate solution
A technology of cholinesterase and substrate solution, applied in the field of determining cholinesterase activity and determining the solution field of cholinesterase activity, can solve problems such as inability to obtain, low efficiency, time-consuming and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Determining cholinesterase activity
[0050] Cholinesterase (CHE) activity was determined based on the formation and detection of thiocholine according to the following reaction scheme:
[0051]
[0052] 2 Thiocholine Dithiobis(choline)
[0053] +2OH - +[Fe(CN) 6 ] 3- ——→+H 2 O+2[Fe(CN) 6 ] 4-
[0054] Thiocholine is specifically released from butyrylthiocholine depending on cholinesterase activity. Thiocholine rapidly reduces yellow hexacyanoferrate III to nearly colorless hexacyanoferrate II. The reduction in extinction can be measured spectrophotometrically at a wavelength of 405 nm and a temperature of 37°C. By the molar absorption coefficient of potassium hexacyanoferrate III (92.7+ / -0.4m 2 / mol) to calculate the concentration of cholinesterase.
[0055] Specifically, the cholinesterase activity was determined as follows:
[0056] Two reagent solutions, R1 and R2, are freshly prepared:
[0057] R1: 91.5mM pyrophosphate
[0058] 2.44mM potassiu...
Embodiment 2
[0102] Evaluation of the Stability of Butyrylthiocholine (BTC) Using Different Polar Organic Solvents
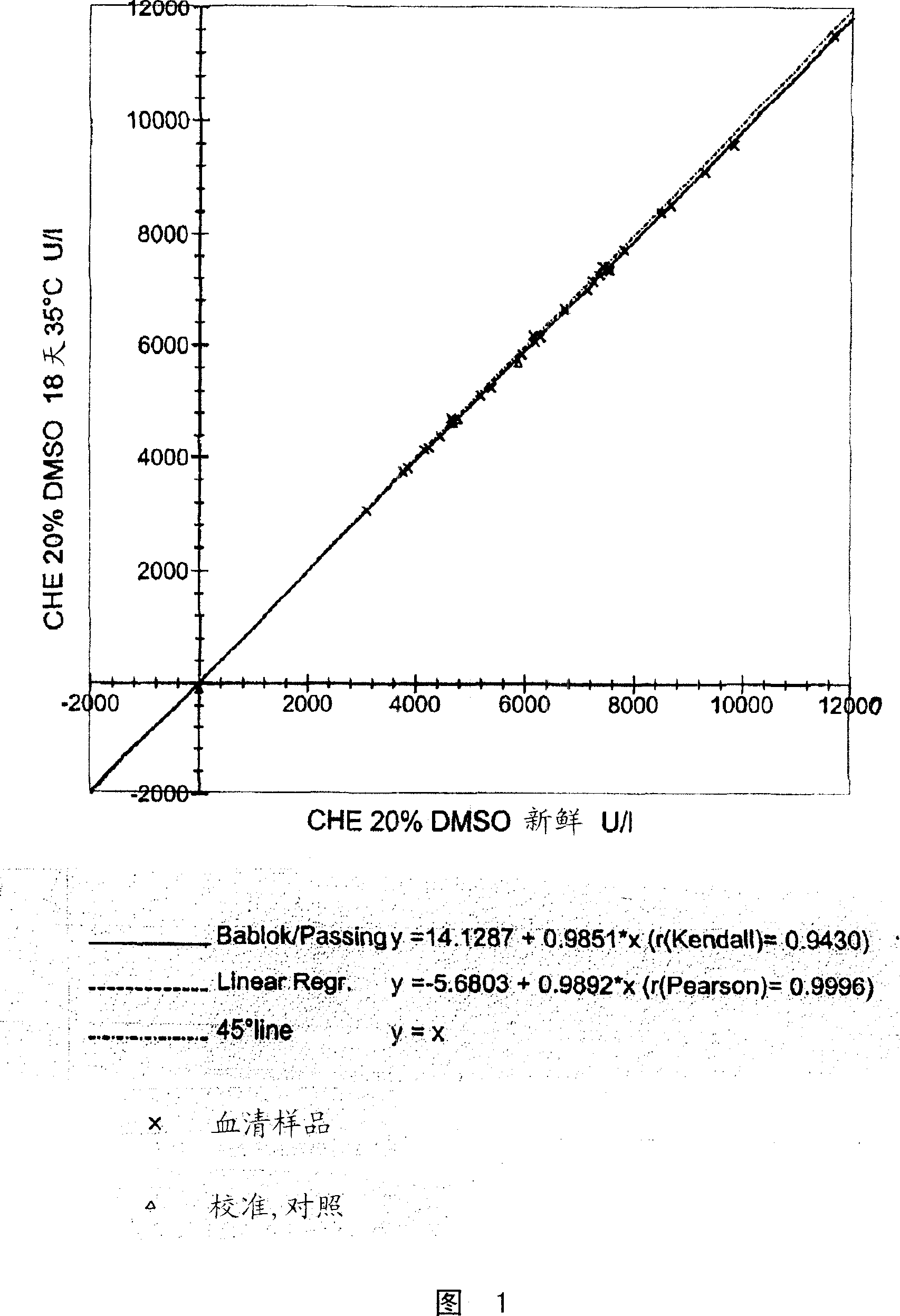
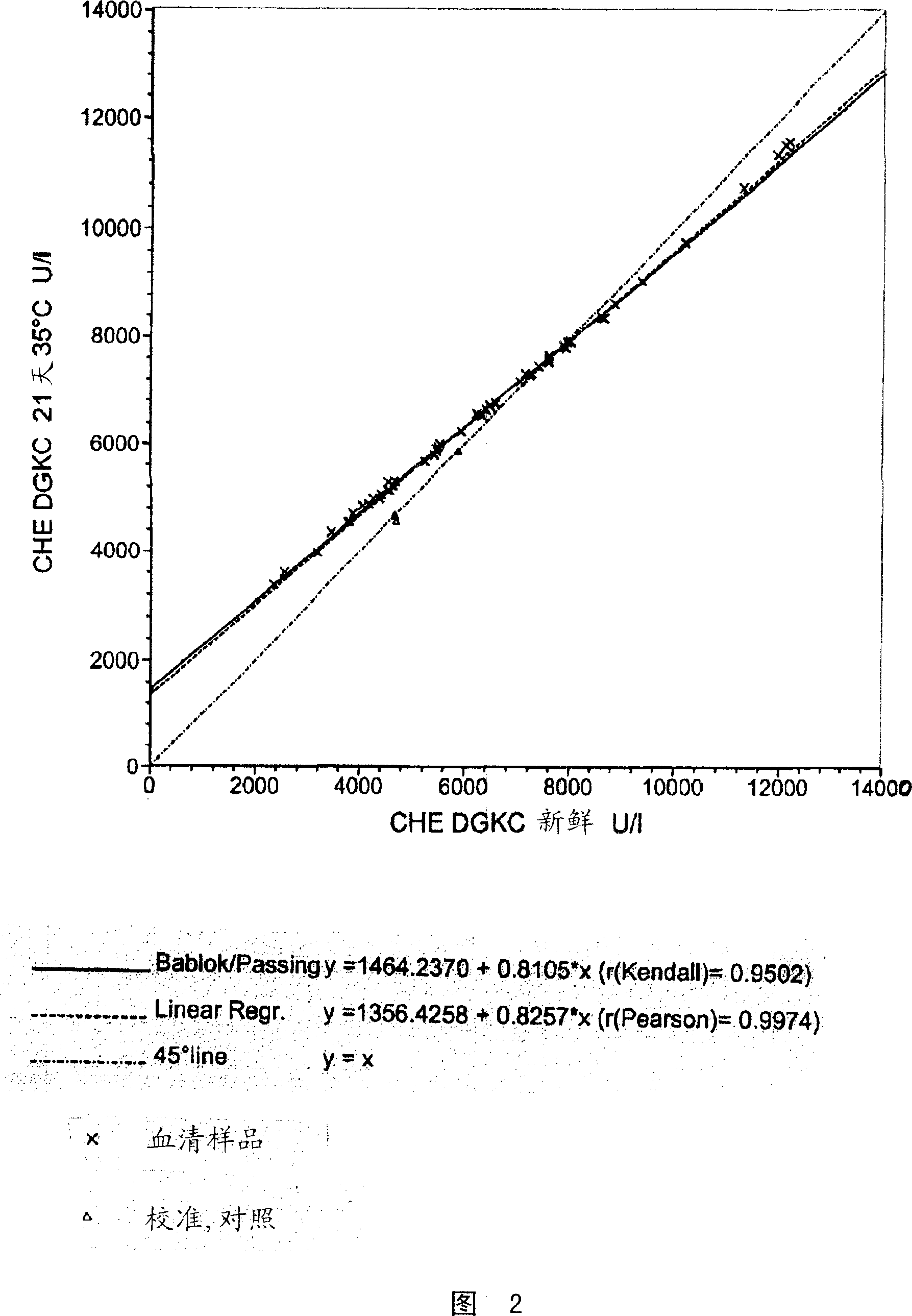
[0103]Insufficient stabilization of BTC can be seen in the variation of reagent aging results. To assess BTC stability, the catalytic concentration of cholinesterase obtained from a temperature-controlled substrate solution (at 35°C for 18-21 days) was compared with the catalytic concentration of cholinesterase obtained from a freshly prepared substrate solution. concentration for comparison.
[0104] Measurements were performed on a Roche / Hitachi 917 analyzer similar to the procedure described in Example 1.
[0105] The above "aging model" is suitable for rapid evaluation of different cholinesterase substrate solutions, because the reagents at 35°C for 18-21 days are equivalent to the reagents stored under normal conditions of refrigeration for 15-18 months.
[0106] Using this "aging model", the stability of different cholinesterase substrate solutions - / + certain polar ...
Embodiment 3
[0121] Determination of Limiting Concentrations of Different Polar Organic Solvents for the Stability of Butyrylthiocholine (BTC)
[0122] Based on the "aging model" described in Example 2, the minimum concentration of polar organic solvent (in volume percentage).
[0123] Table 2: Stability of butyrylthiocholine (BTC)
[0124] And determine the limit concentration of different polar organic solvents.
[0125] ethanol
[0126] Considering the criteria for stabilizing the cholinesterase substrate solution, i.e. the intercept is about less than + / -300 U / l and the slope is less than + / -3%, the lowest concentration with a stabilizing effect is 1% (vol. %), 2% (vol%) for THF and isopropanol, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com