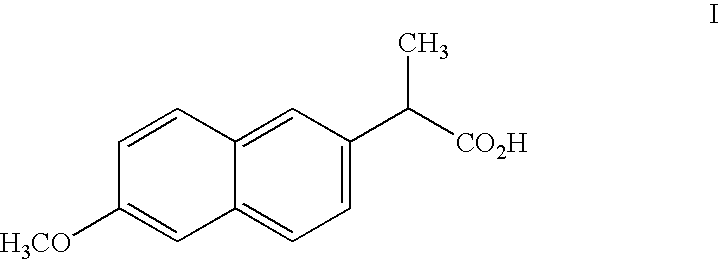
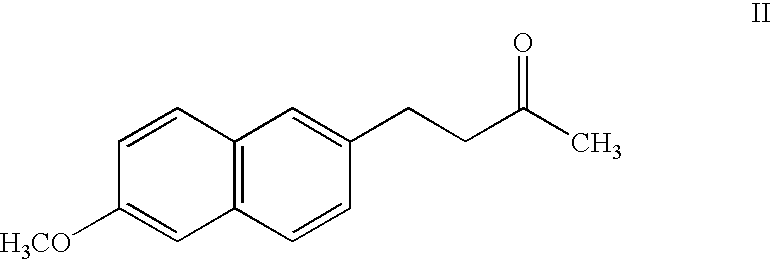
6-Methoxy-2-naphthylacetic acid prodrugs
a technology of naphthylacetic acid and prodrugs, which is applied in the direction of biocide, amide active ingredients, drug compositions, etc., can solve the problems of severe irritation of the gastronintestinal tract at dosage, stomach irritation and/or ulceration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
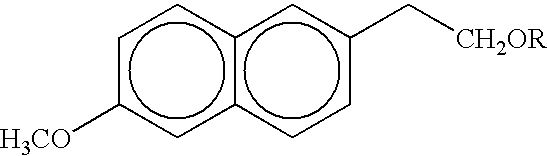
[0039] Synthesis of 2-(6-Methoxy-naphthalen-2-yl)-ethanol
[0040] 6-MNA (1g, 0.0046 mol) was suspended in anhydrous THF and was cooled with ice bath suspension BH3(1 M solution in THF, 5 ml) was added. The reaction mixture was stirred for 3 hours then Dl water and sodium carbonate added. THF was removed and aqueous phase extracted with ethyl acetate then washed with water, dried over Na2SO4, filtered concentrated and dried via vacuum.
[0041] Yield 93%. Melting point 110-113.degree. C. product was analyzed by elemental analysis, IR, MS, NMR.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
| in plasma levels | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com