Apoptosis-mimicking synthetic entities and use thereof in medical treatment
a synthetic entity and apoptosis technology, applied in the field of synthetic and semi-synthetic compositions having biochemical activity, can solve the problems of large amount of liposomes given to a human, too large to act as immune system modifiers, and high cost and therefore undesirabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-5
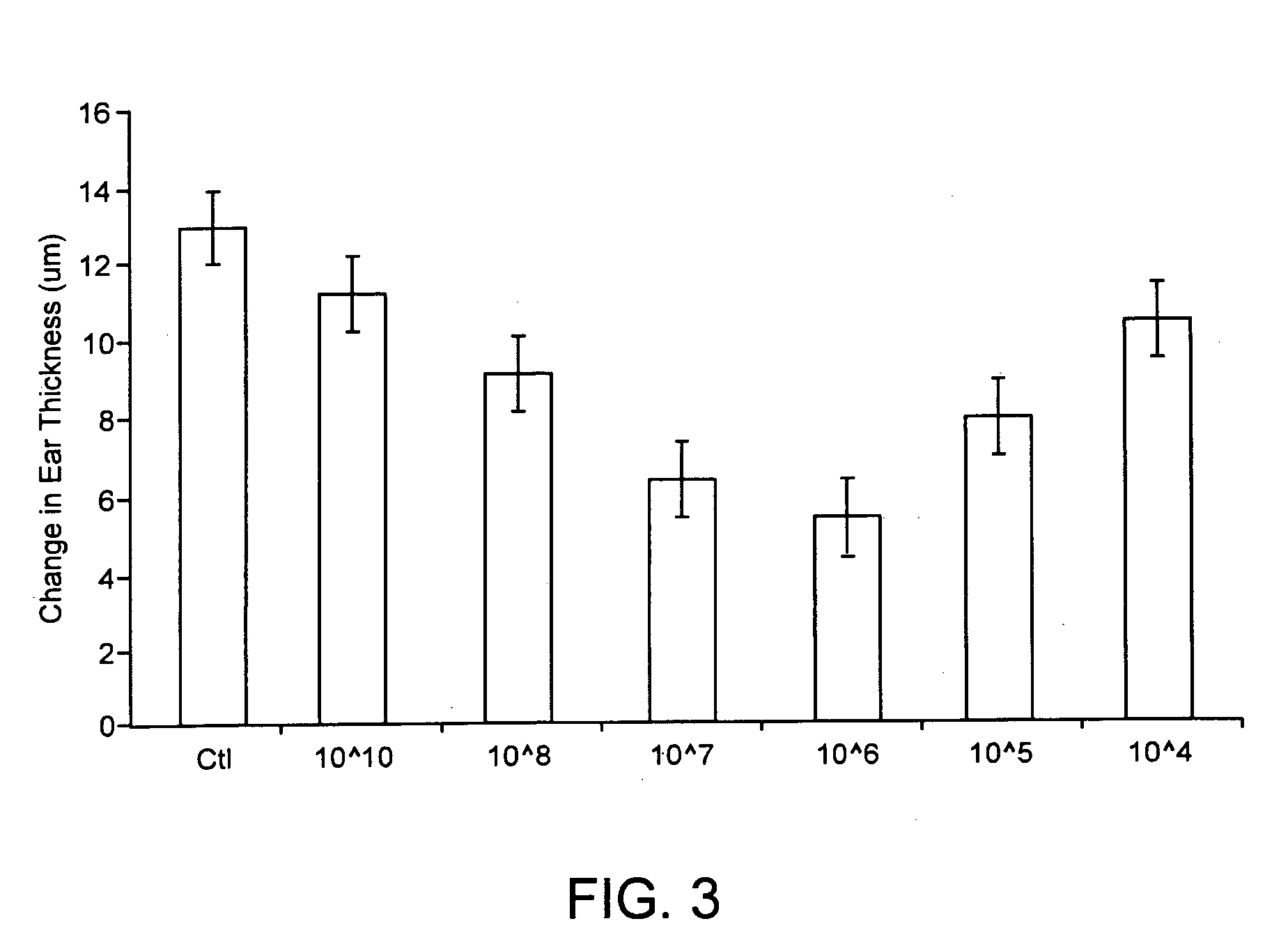
[0064] These examples show the effect of injecting various phosphatidylserine-containing liposomes of various sizes and compositions, and in various concentrations, on ear swelling in the murine contact hypersensitivity (CHS) model, a Th1-mediated inflammatory reaction, an art-accepted model. For these experiments, female BALB / c mice (Jackson Laboratories) age 6-8 weeks and weighing 22-25 g were used.
example 1
PS Liposomes
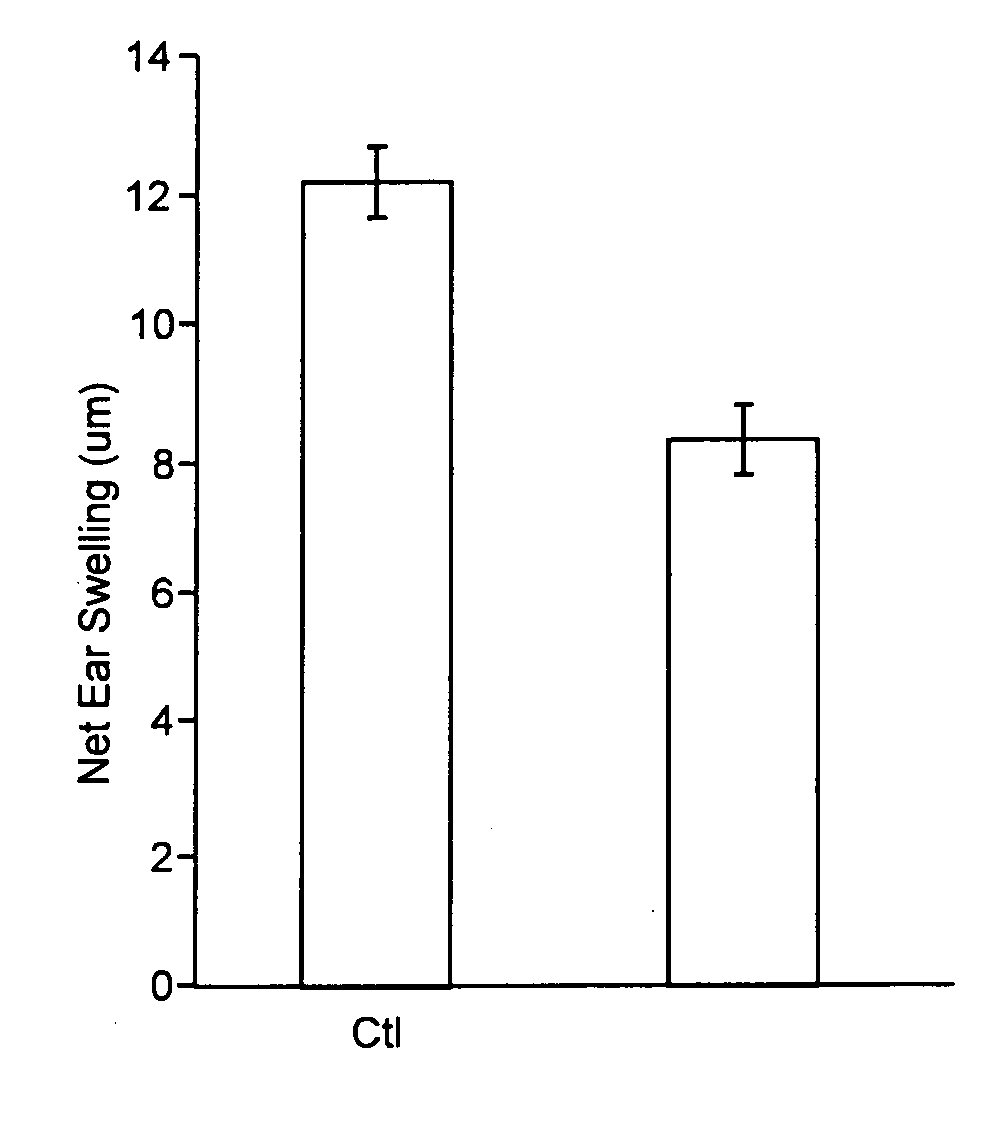
[0065] Phosophatidylserine (PS) containing liposomes of size 100±20 nanometers were prepared as described above which had a phosphatidylserine content of 100% (i.e. made entirely of PS). A stock suspension of liposomes containing 4.8×1014 liposomes per ml was diluted with PBS to give an injection suspension containing 6×106 particles per ml, and each animal was injected with 50 μl of this suspension, containing 3×105 liposomes.
Protocol
[0066] Test group A which received the liposomes comprised 25 animals in number. Control group comprised 24 animals. The following experiments were run:
TABLE 1Day 7GroupDay 1Day 2Day 3Day 4Day 5Day 6(24 hours)AInjectInjectInjectInjectInjectInjectEar measured100%thenthenPSsensitizechallengepositivesensitizedchallengeEar measuredcontrol(untreatedanimals)
[0067] On Days 1-6, mice of Group A were injected with liposomes prepared from 100% PS. Liposomes were injected in 50 μl volume via intramuscular (IM) injection, i.e. 300,000 liposomes pe...
example 2
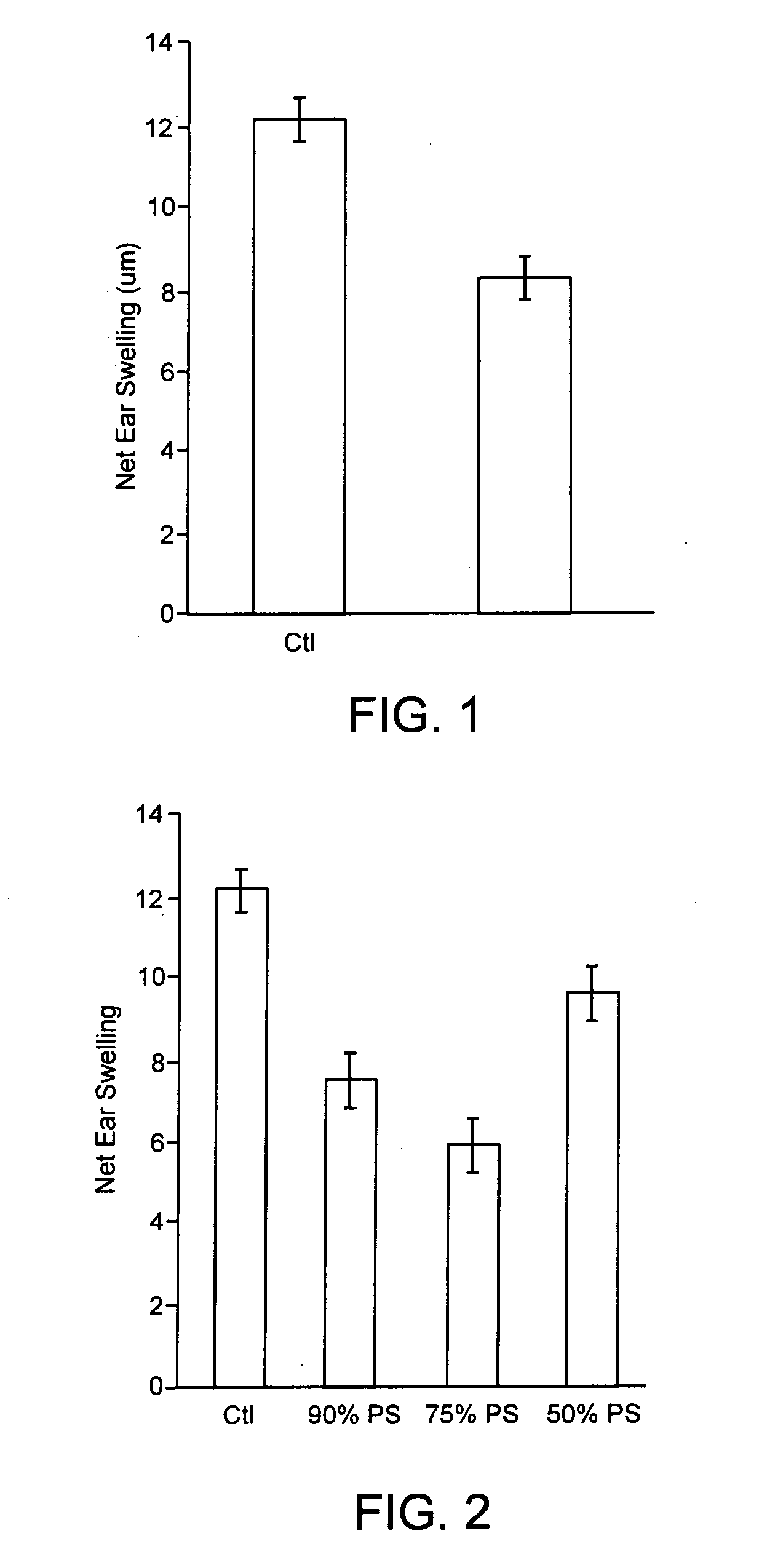
[0071] Liposomes of size 100±20 nanometers and containing different amounts of phosphatidyl serine, namely 90%; 75% and 50%, with the remainder being phosphatidylcholine (PC), were prepared as described, from starting mixtures of the appropriate relative quantities of the respective phospholipids. Using 4 different groups of animals, five mice per group, the following experiments were run.
Day 7GroupDay 1Day 2Day 3Day 4Day 5Day 6(24 hours)BInjectInjectInjectInjectInjectInjectEar measured90% PSthenthensensitizechallengeCInjectInjectInjectInjectInjectInjectEar measured75% PSthenthensensitizechallengeDInjectInjectInjectInjectInjectInjectEar measured50% PSthenthensensitizechallengepositivesensitizedchallengedEar measuredcontrol(untreatedanimals
The amounts and procedure for injection, the sensitization procedure, the challenge procedure and the results measurements were all as described in Example 1.
[0072] The results are given below, in Table 2, and are graphically presented on FIG. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com