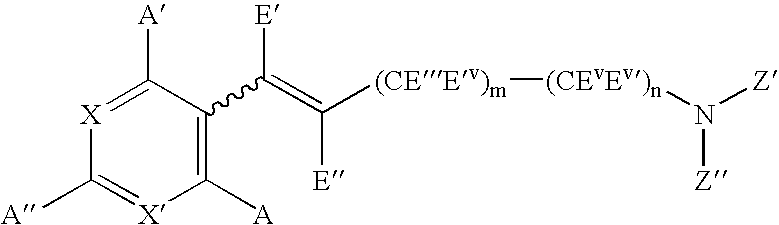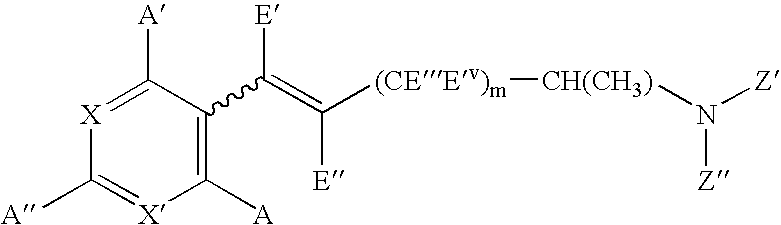Compounds capable of activating cholinergic receptors
a technology compounds, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, biocides, etc., can solve the problems of not providing significant side effects, and achieve the effects of increasing the number of nicotinic cholinergic receptors of the brain of the patient, preventing and suppressing symptoms, and increasing blood pressure and heart ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Determination of Log P Value
[0038] Log P values, which have been used to assess the relative abilities of compounds to pass across the blood-brain banrier (Hansch, et al., J. Med. Chem. ii:1 (1968)), were calculated using the Cerius2 software package Version 3.5 by Molecular Simulations, Inc.
example 2
Determination of Binding to Relevant Receptor Sites
[0039] Binding of the compounds to relevant receptor sites was determined in accordance with the techniques described in U.S. Pat. No. 5,597,919 to Dull et al. Inhibition constants (Ki values), reported in nM, were calculated from the IC50 values using the method of Cheng et al., Biochem, Pharmacol. 22:3099 (1973).
example 3
Determination of Dopamine Release
[0040] Dopamine release was measured using the techniques described in U.S. Pat. No. 5,597,919 to Dull et al. Release is expressed as a percentage of release obtained with a concentration of (S)-(−)-nicotine resulting in maximal effects. Reported EC50 values are expressed in nM, and Emax values represent the amount released relative to (S)-(−)-nicotine on a percentage basis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com