Seed coat specific DNA regulatory region and peroxidase
a technology of dna regulatory region and seed coat, which is applied in the direction of enzymes, plant cells, chemical libraries, etc., can solve problems such as variability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
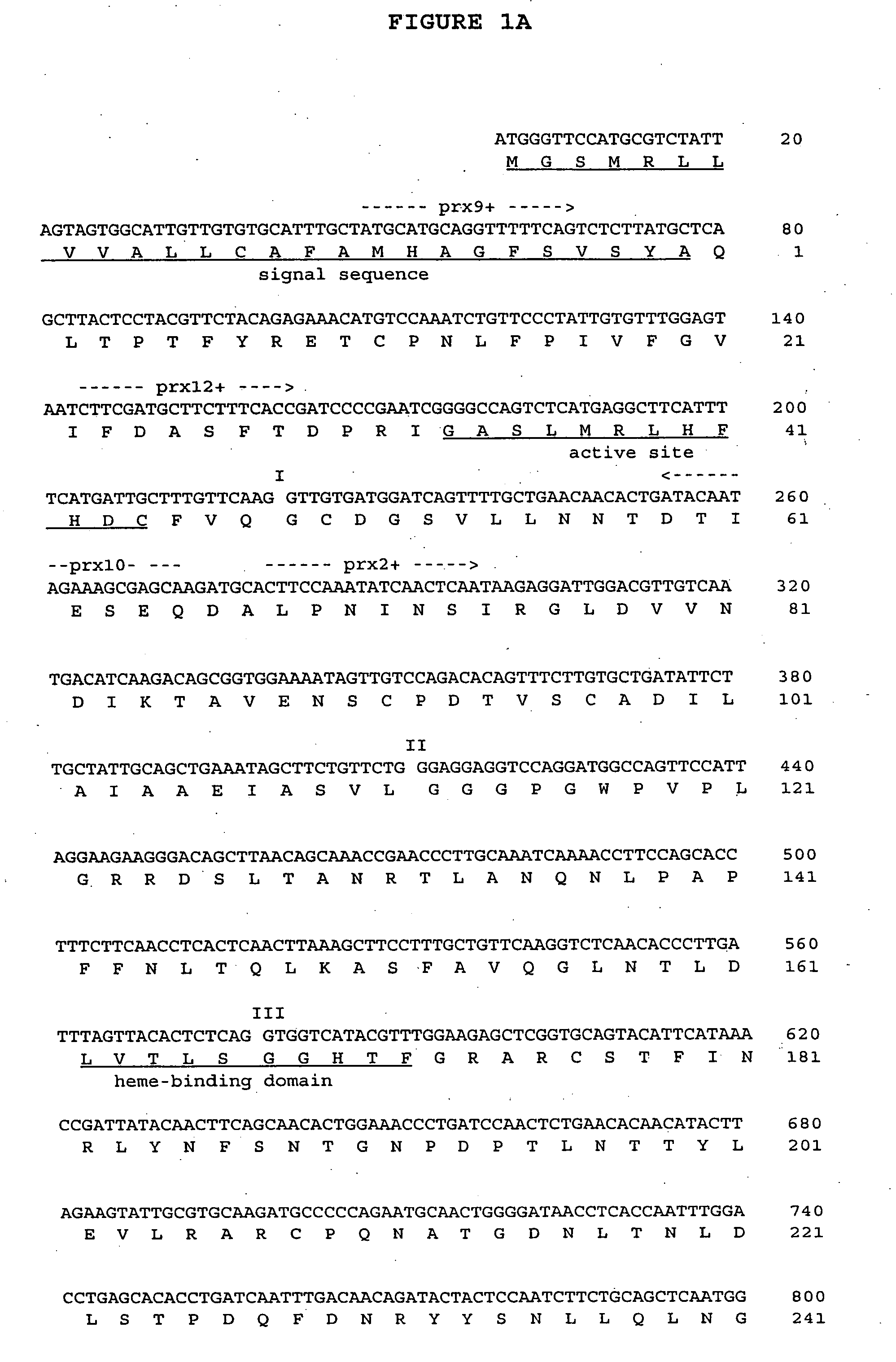
The Seed Coat Peroxidase cDNA and Genomic DNA Sequences
[0082] To isolate the seed coat peroxidase transcript, a cDNA library was constructed from developing seed coat tissue of the EpEp cultivar Harosoy 63. The primary library contained 106 recombinant plaque forming units and was amplified prior to screening. A degenerate 17-mer oligonucleotide corresponding to the conserved active site domain of plant peroxidases was used to probe the library. In screening 10,000 plaque forming units, 12 positive clones were identified. The cDNA insert size of the clones ranged from 0.5 to 2.5 kb, but six clones shared a common insert size of 1.3 kb. These six clones (soyprx03, soyprx05, soyprx06, soyprx11, soy prx12, and soyprx14) were chosen for further characterization since the 1.3 kb insert size matched the expected peroxidase transcript size. Sequence analysis of the six clones showed that they contained identical cDNA transcripts encoding a peroxidase and that each resulted from an indepen...
example 2
DNA Blot Analysis Using the Seed Coat Peroxidase cDNA Probe Reveals Restriction Fragment Length Polymorphisms Between EpEp and epep Genotypes
[0088] Genomic DNA blots of OX347 (EpEp) and OX312 (epep) plants were hybridized with 32P-labelled cDNA to estimate the copy number of the seed coat peroxidase gene and to determine if this locus is polymorphic between the two genotypes. FIG. 4 shows the hybridization patterns after digestion with BamHI, XbaI, and SacI. Restriction fragment length polymorphisms are clearly visible in the BamHI and SacI digestions. The BamHI digestion produced a strongly hybridizing 17 kb fragment and a faint 3.4 kb fragment in the EpEp genotype. The 3.4 kb BamHI fragment is visible in the epep genotype but the 17 kb fragment has been replaced by a signal at >20 kb. The SacI digestion resulted in detection of three fragments in EpEp and epep plants. At least two fragments were expected here since the cDNA sequence has a SacI site within the open reading frame. ...
example 3
A Deletion Mutation Occurs in the Recessive ep Locus
[0090] The structural gene encoding the seed coat peroxidase is schematically illustrated in FIG. 5. The 17 kb BamHI fragment encompassing the gene includes 191 bp of sequence upstream from the translation start codon, three introns of 631 bp, 1030 bp, and 263 bp, and 13 kb of sequence downstream from the polyadenylation site. The arrangement of four exons and three introns and the placement of introns within the sequence is similar to that described for other plant peroxidases (Simon, 1992; Osakabe et al. 1995).
[0091] Primers were designed from the DNA sequence to compare EpEp and epep genotypes by PCR analysis. FIG. 6 shows PCR amplification products from four different primer combinations using OX312 (epep) and OX347 (EpEp) genomic DNA as template. The primer annealing site for prx29+begins 182 bp upstream from the ATG start codon; the remaining primer sites are shown in FIG. 1. Amplification with primers prx2+ and prx6−, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap