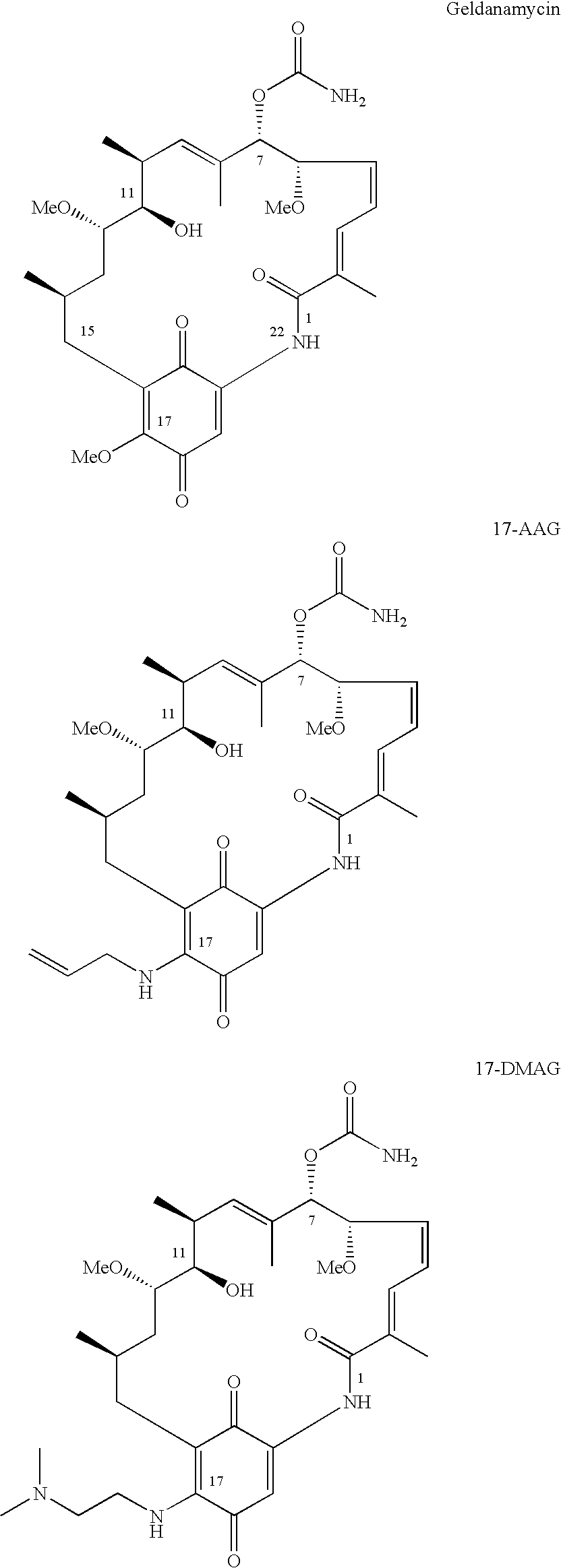
Pharmaceutical solution formulations containing 17-AAG
a technology of pharmaceutical solution and 17-aag, which is applied in the field of pharmaceutical solution formulations, can solve the problems of reducing the sensitivity of proteasome-mediated destruction of aag, discontinuing clinical candidates, and poor water solubility of aag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0036] This example describes the preparation of a vehicle for use in formulations of this invention, consisting of 50 volume % ethanol, 20 volume % Cremophor EL, and 30 volume % propylene glycol. Dehydrated ethanol (USP, 500 mL, 394.5 g) was mixed with Cremophor EL (BASF Aktiengesellschaft, 200 mL, 210 g). After the foregoing two components were mixed to form a homogeneous liquid, propylene glycol (USP, 300 mL, 310.8 g) was added. The combination was mixed again to homogeneity and filtered through a 0.22μ filter, to provide 1 liter of vehicle.
example 2
[0037] Following the general procedure of Example 1, another 1 L-batch of vehicle was prepared, using 450 mL (355.1 g) of ethanol, 280 mL (294 g) of Cremophor EL, and 270 mL (279.5 g) of propylene glycol. This resulted in a vehicle consisting of 45 volume % ethanol, 28 volume % Cremophor EL, and 27 volume % propylene glycol.
example 3
[0038] Following the general procedure of Example 1, additional 1 L-batches of vehicle were prepared, using 500 mL (394.5 g) of ethanol and 150 to 500 mL (157.5 to 525 g) of Cremophor EL, and 0 to 350 mL propylene glycol. This resulted in vehicles consisting of 50 volume % ethanol, 15 to 50 volume % Cremophor EL, and 0 to 35 volume % propylene glycol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume % | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| volume % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com