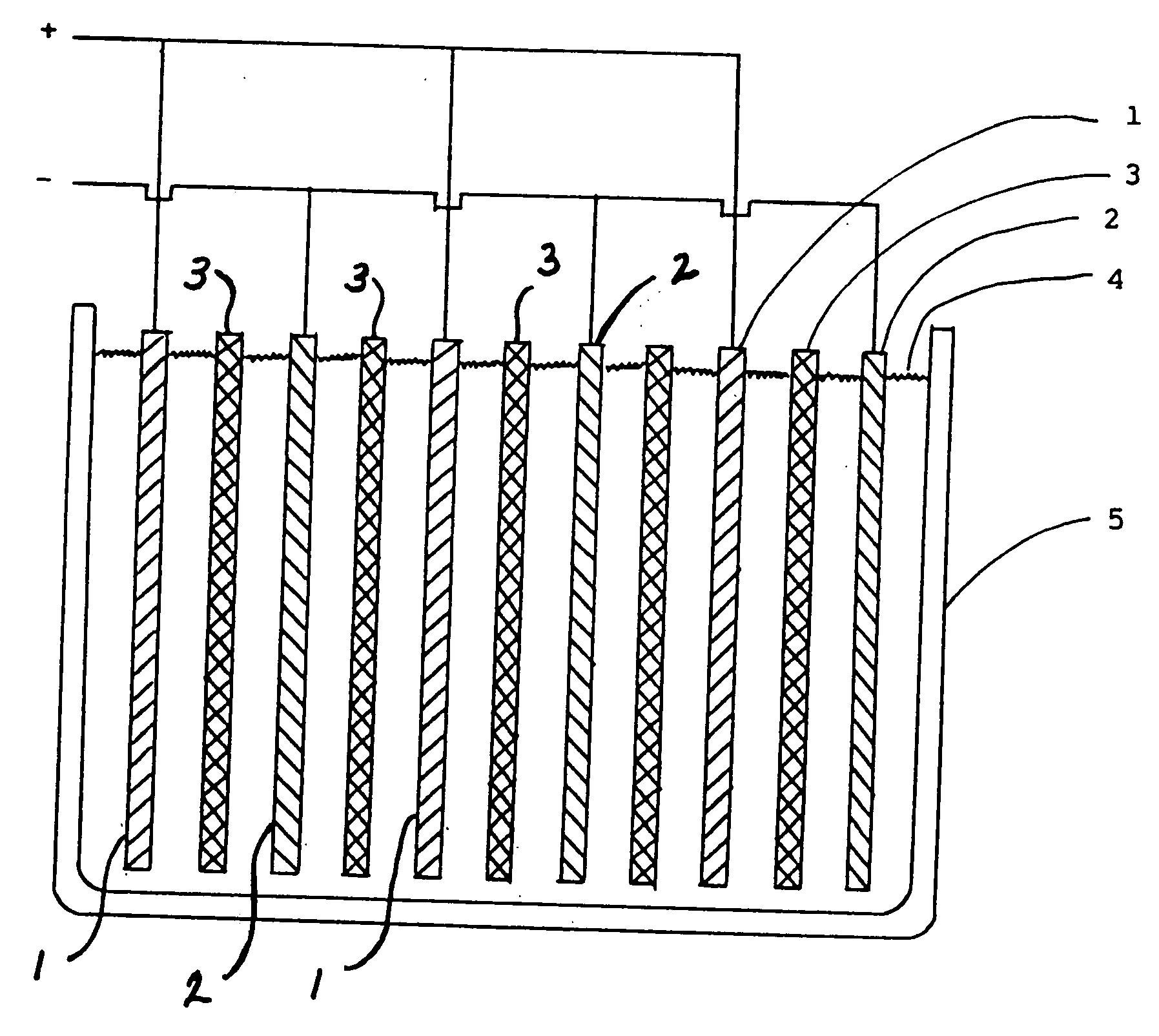
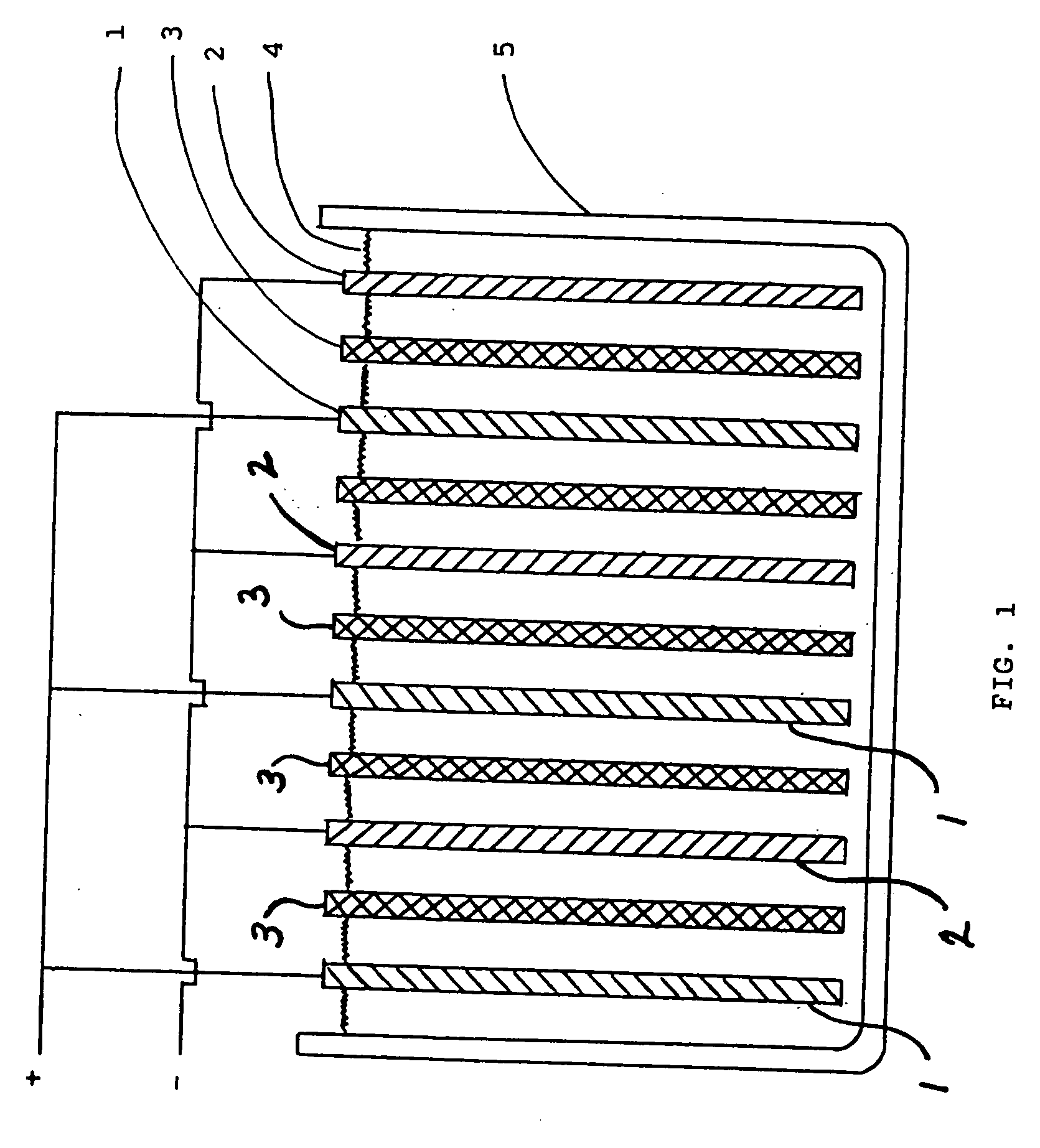
Lead-zinc battery
a technology of lead zinc and storage batteries, applied in the direction of lead-acid accumulators, cell components, electrochemical generators, etc., can solve the problems of limited energy density, limited cycle life, and extremely corrosive electrolyte, and achieve superior electrical conductivity, improve cell capacity, and improve performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] 1. The electrolyte in this example contained sodium carbonate. The sodium carbonate solution was prepared by heating 96.8 g. of baking soda (sodium bicarbonate) to 500° F. and dissolving the product in 185 ml. of water. The positive electrode was formed from a 1½ in. wide strip of lead obtained from a plumbing supply business. The negative electrode was a 1½ in. wide strip of zinc which had been removed from a flashlight battery of the Leclanche type. The cell compromised a glass jar about 2¾ in. diameter by 2½ in. high. After charging the cell for 23 minutes at 2.50 v., an open circuit voltage of 2.32 v. was observed for the cell. The cell was repeatedly discharged and charged during the course of the run. At the end of the experiment, the electrodes were in perfect condition and the electrolyte was water-white. [0025] 2. In this run the electrolyte was a solution of potassium borate. The same electrodes and container were used as in example 1. To prepare the electrolyte 40....
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy density | aaaaa | aaaaa |
| energy densities | aaaaa | aaaaa |
| alkalinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com