Universal stem cells
a stem cell and universal technology, applied in the field of universal stem cells, can solve the problems of further complexity, cell effect, and the possibility of haplotype- and isotype-mismatched dimers, and achieve the effect of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
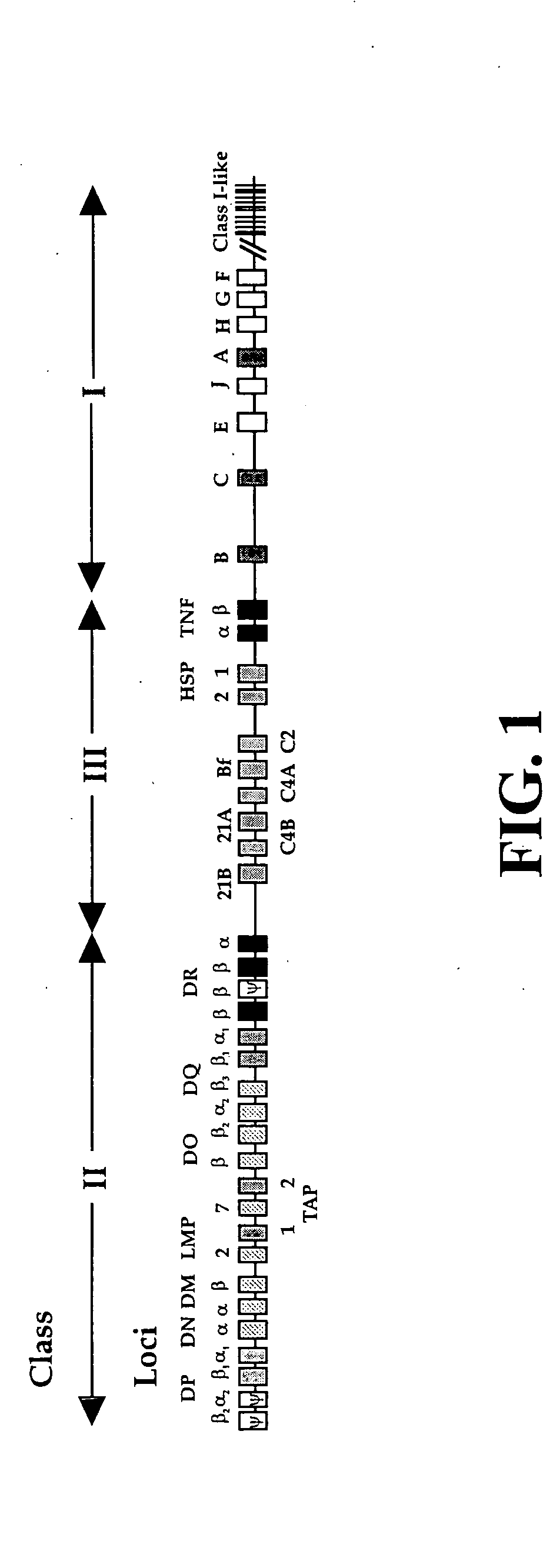
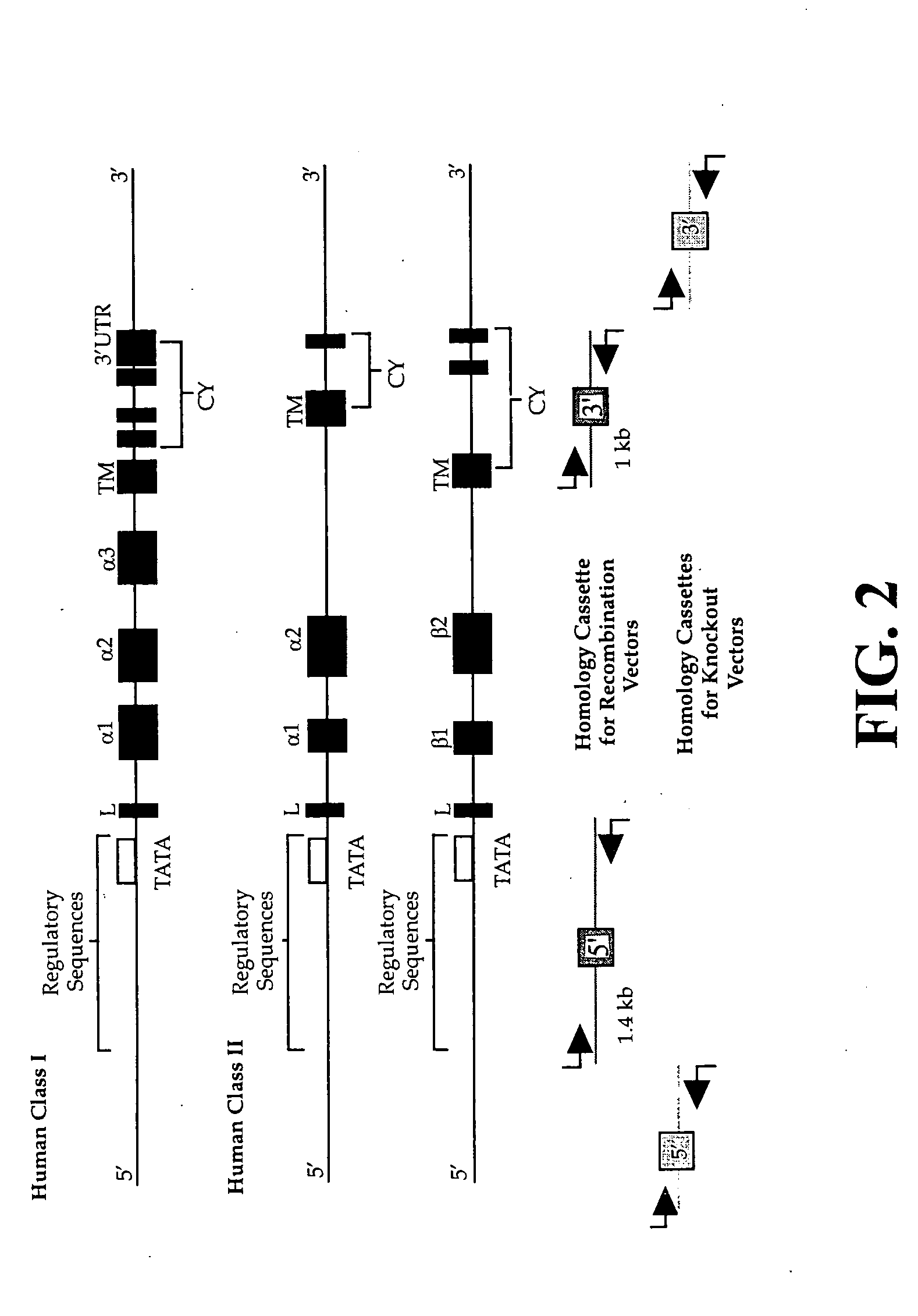
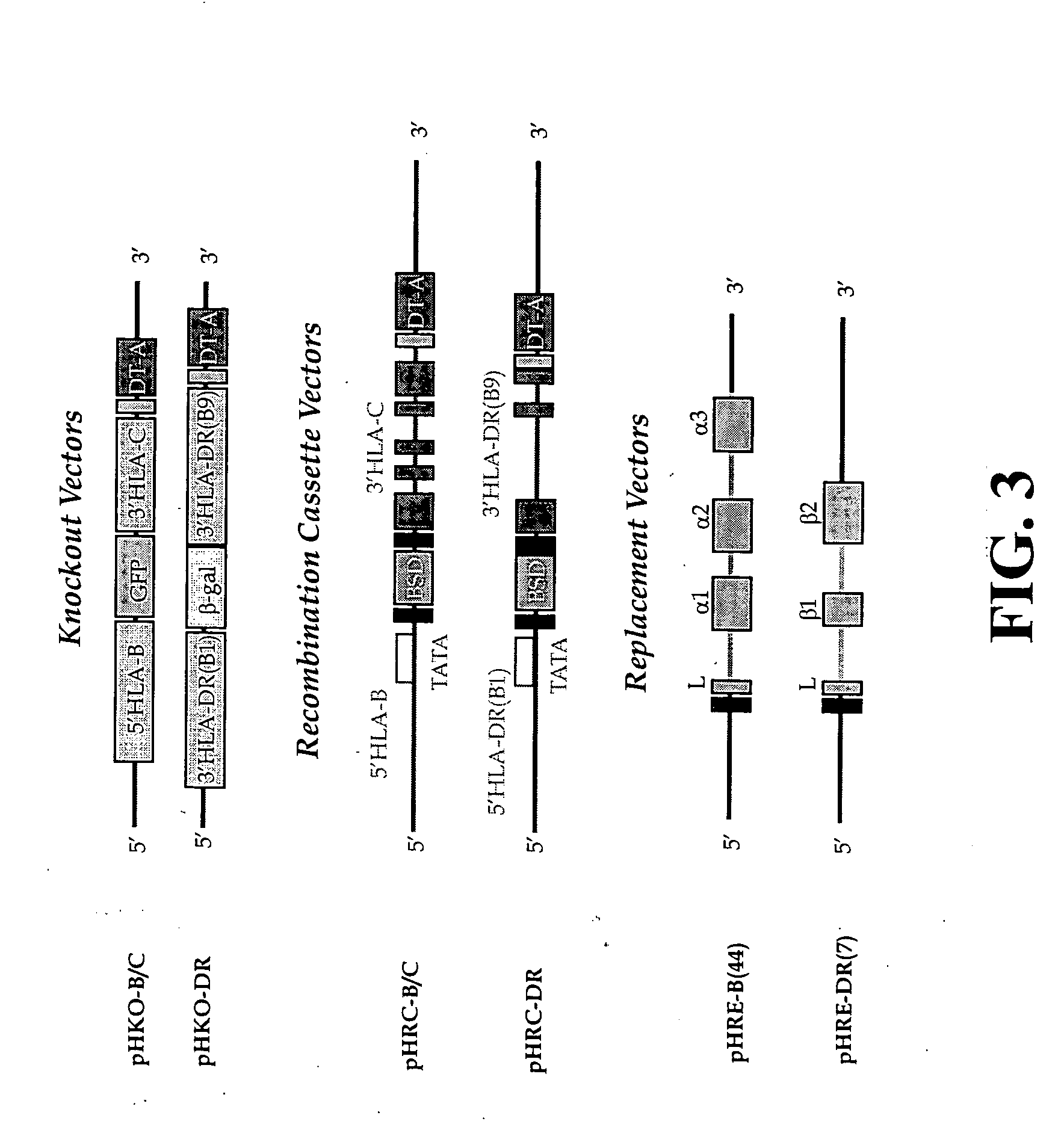
[0035] The subject invention concerns materials and methods for preparing cells expressing a pre-selected or heterologous histocompatibility gene or allele. Typically, these cells are multi-potential stem cells. Cells of the subject invention can be used to generate compatible tissues / organs for transplantation. One embodiment of the process of the subject invention comprises the use of targeting vectors capable of gene knockout, insertion of site-specific recombination cassettes, and the replacement of histocompatibility alleles. As used herein, the term “pre-selected” means that the chosen gene or allele to be integrated into a chromosome of the cell is one that is not normally expressed by the unaltered cell.
[0036] The subject invention also concerns novel vectors used to prepare cells of the invention. Encompassed within the scope of the invention are knockout vectors, recombination cassette vectors, and replacement vectors for use in human and mammalian cells. Specifically exe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adhesion | aaaaa | aaaaa |
| polymorphic | aaaaa | aaaaa |
| tertiary structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com