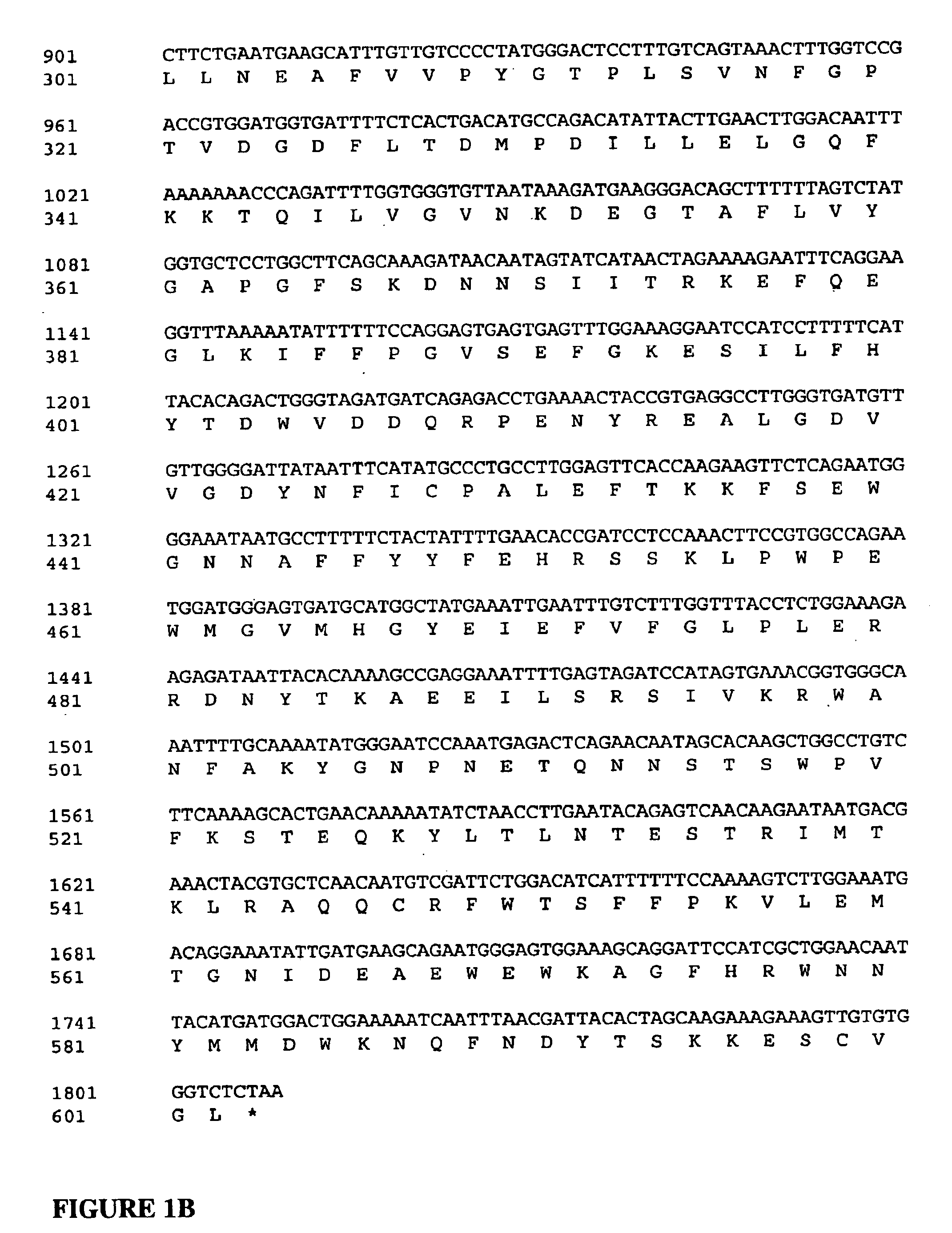
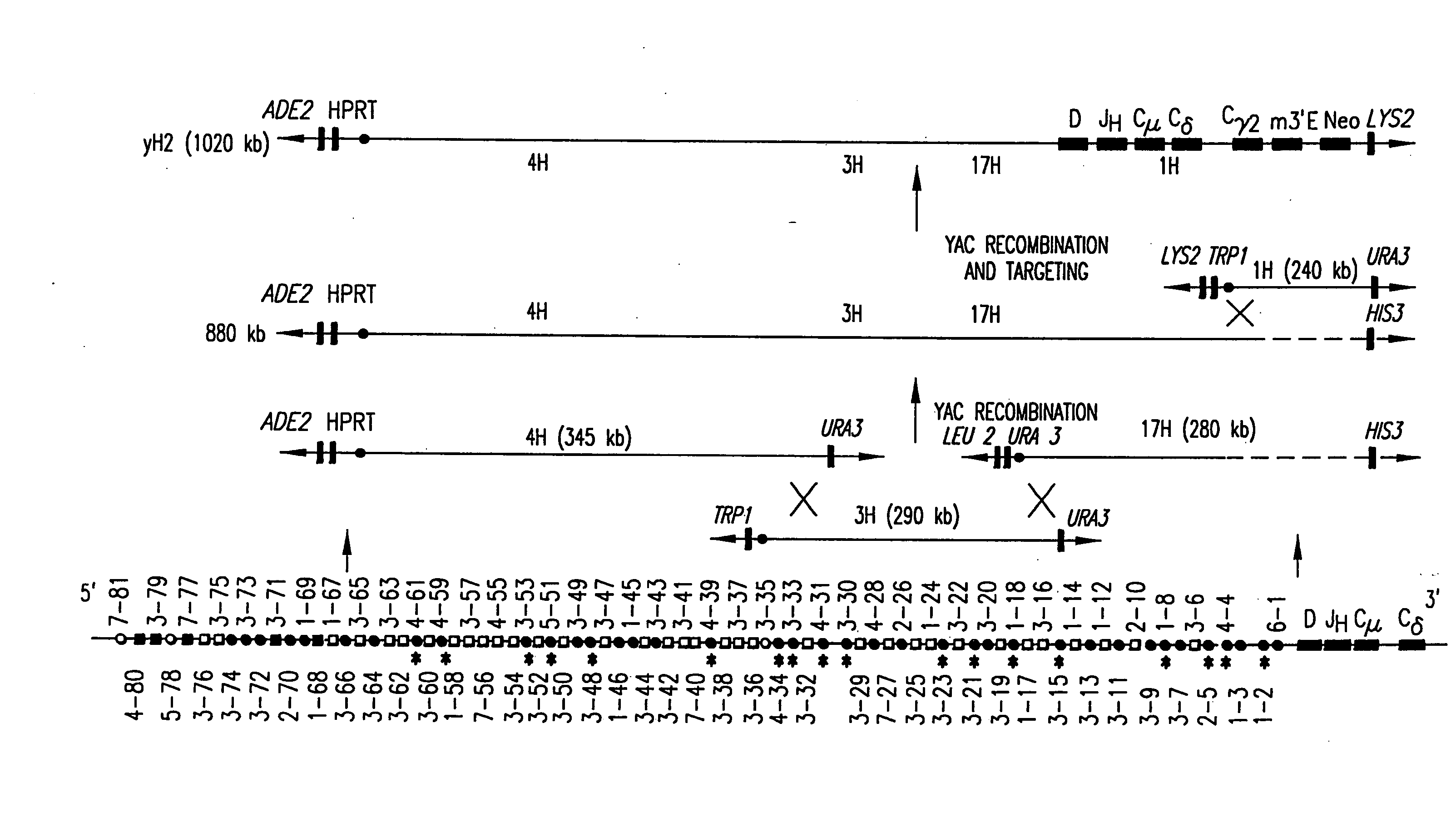
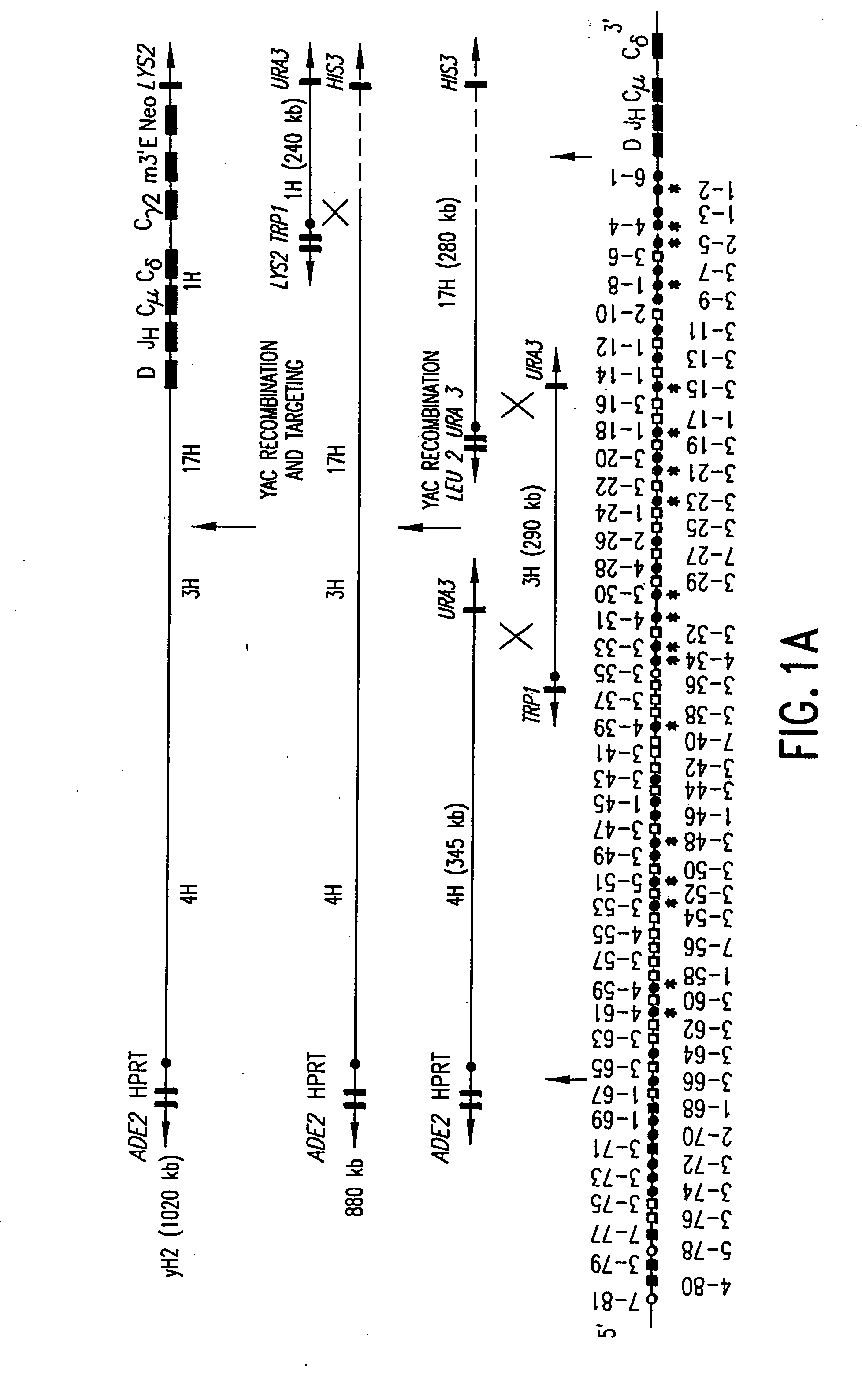
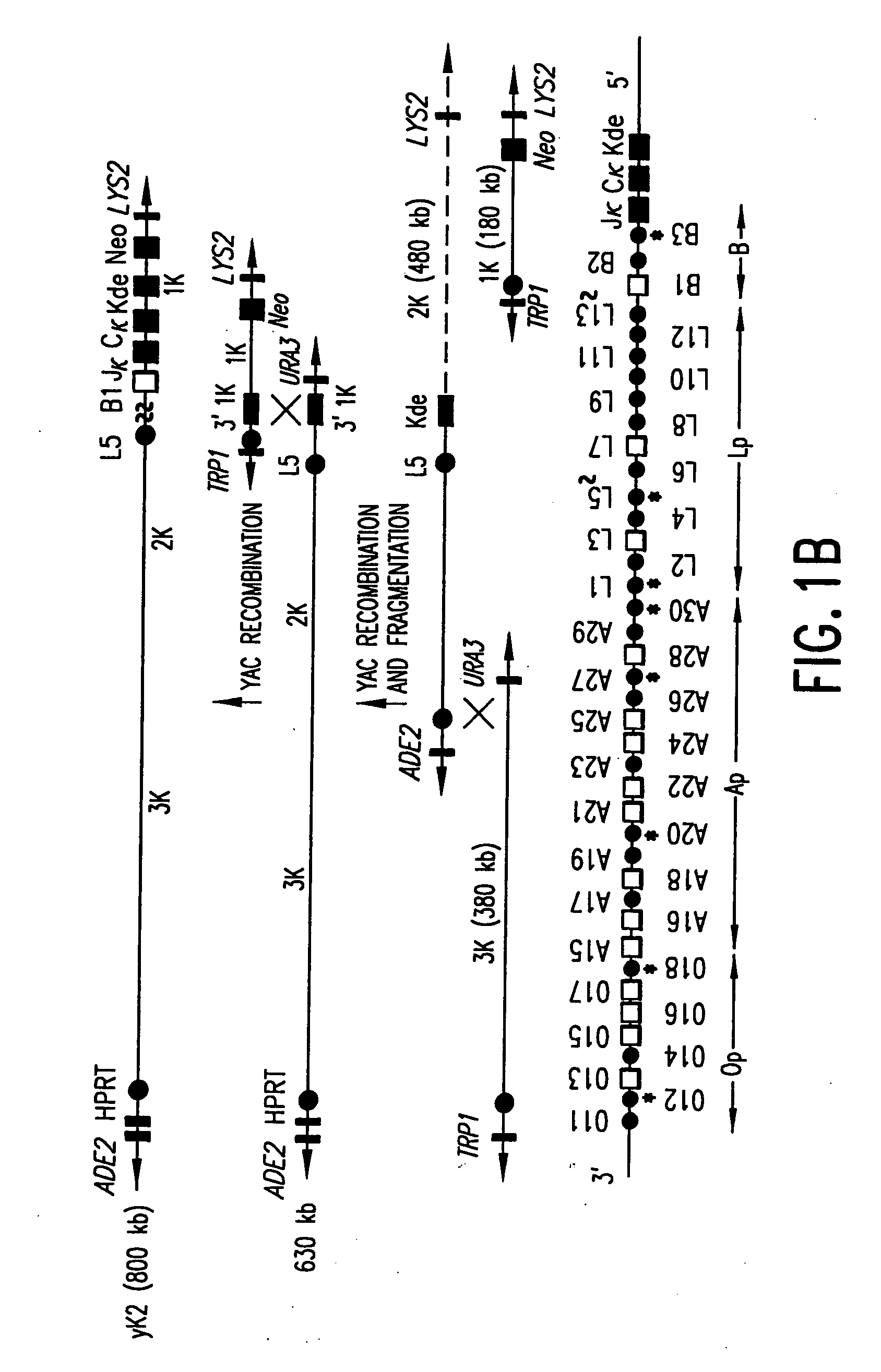
Patents
Literature
143 results about "Genetically modified mammal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Genetically modified mammals are mammals that have been genetically engineered. They are an important category of genetically modified organisms. The majority of research involving genetically modified mammals involves mice with attempts to produce knockout animals in other mammalian species limited by the inability to derive and stably culture embryonic stem cells.
Transgenic mammals having human Ig loci including plural VH and VK regions and antibodies produced therefrom
InactiveUS7064244B2Reduced development and maturation of B-cellsEfficient productionAntipyreticAnalgesicsHuman animalMammal
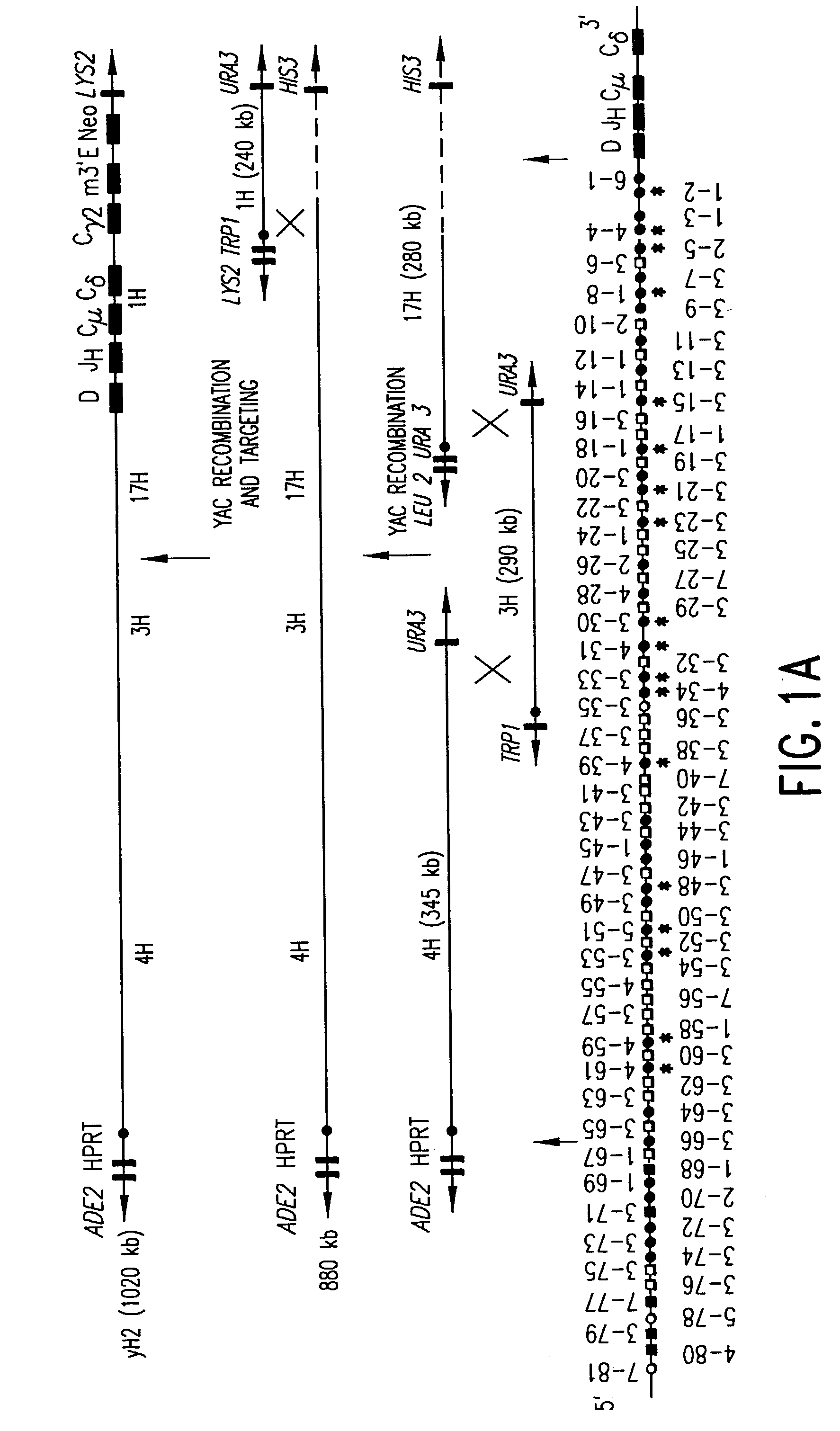
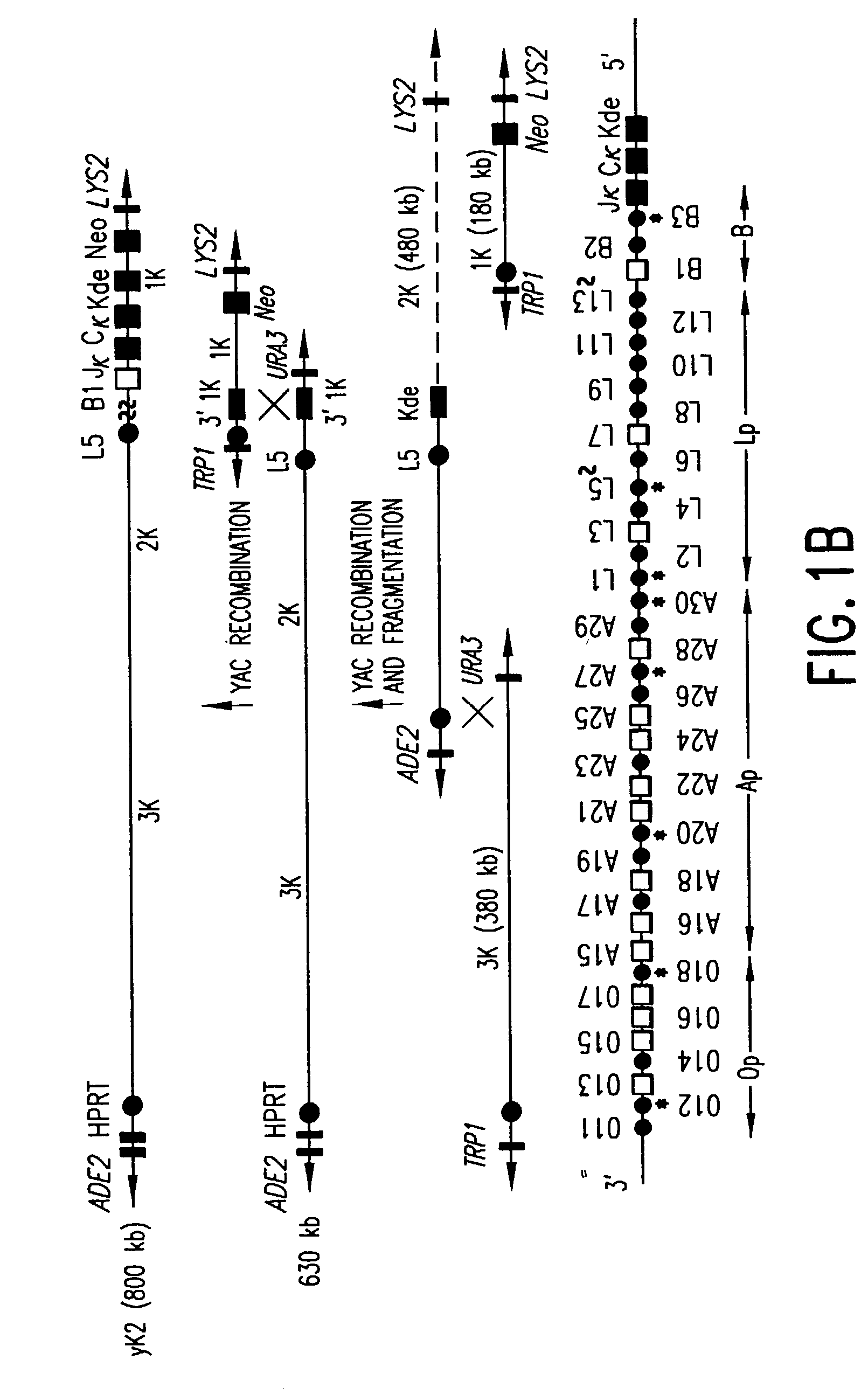
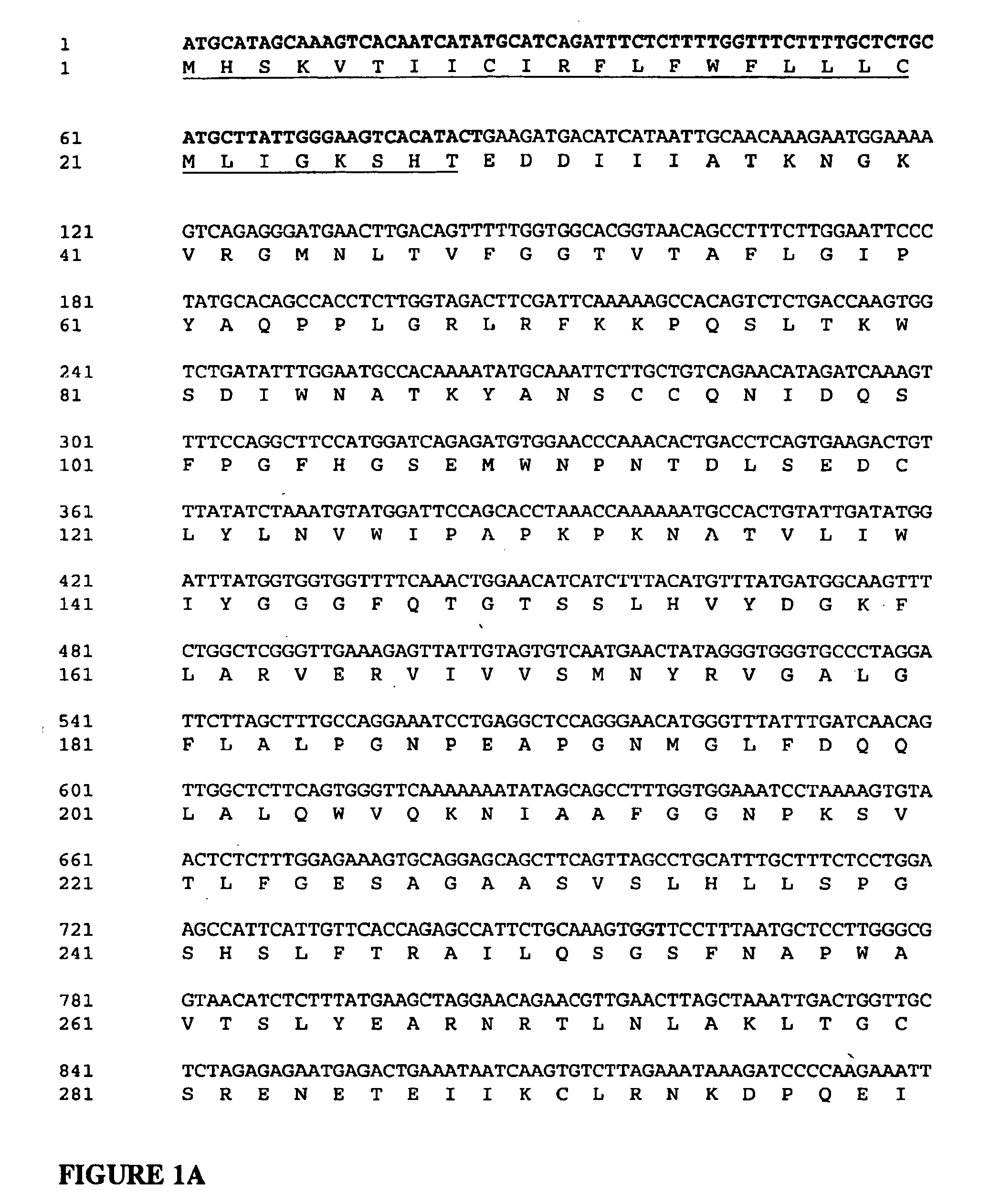
The present invention relates to transgenic non-human animals that are engineered to contain human immunoglobulin gene loci. In particular, animals in accordance with the invention possess human Ig loci that include plural variable (VH and Vκ) gene regions. Advantageously, the inclusion of plural variable region genes enhances the specificity and diversity of human antibodies produced by the animal. Further, the inclusion of such regions enhances and reconstitutes B-cell development to the animals, such that the animals possess abundant mature B-cells secreting extremely high affinity antibodies.
Owner:ABQENIX INC
CICM cells and non-human mammalian embryos prepared by nuclear transfer of a proliferating differentiated cell or its nucleus
InactiveUS6235970B1Simple procedureSimplifying and facilitating procedureNervous disorderMuscular disorderPresent methodNuclear transfer
An improved method of nuclear transfer involving the transplantation of donor differentiated cell nuclei into enucleated oocytes of the same species as the donor cell is provided. The resultant nuclear transfer units are useful for multiplication of genotypes and transgenic genotypes by the production of fetuses and offspring, and for production of isogenic CICM cells, including human isogenic embryonic or stem cells. Production of genetically engineered or transgenic mammalian embryos, fetuses and offspring is facilitated by the present method since the differentiated cell source of the donor nuclei can be genetically modified and clonally propagated.
Owner:UNIVERSITY OF MASSACHUSETTS AMHERST
Production of hSA-linked butyrylcholinesterases in transgenic mammals
InactiveUS20060253913A1Promote formationImprove stabilityAnimal cellsVectorsMammalButyrylcholinesterase
The present invention provides methods for the large-scale production of recombinant butyrylcholinesterase fused to human serum albumin in cell culture, and in the milk and / or urine of transgenic mammals. The recombinant butyrylcholinesterase-albumin fusion protein of this invention can be used to treat and / or prevent organophosphate pesticide poisoning, nerve gas poisoning, cocaine intoxication, and succinylcholine-induced apnea.
Owner:PHARMATHENE
Transgenic mammals having human Ig loci including plural Vh and Vk regions and antibodies produced therefrom
InactiveUS20080098490A1Reduced development and maturation of B-cellsEfficient productionAntipyreticAnalgesicsHuman animalMammal
Owner:AMGEN FREMONT INC
Universal stem cells
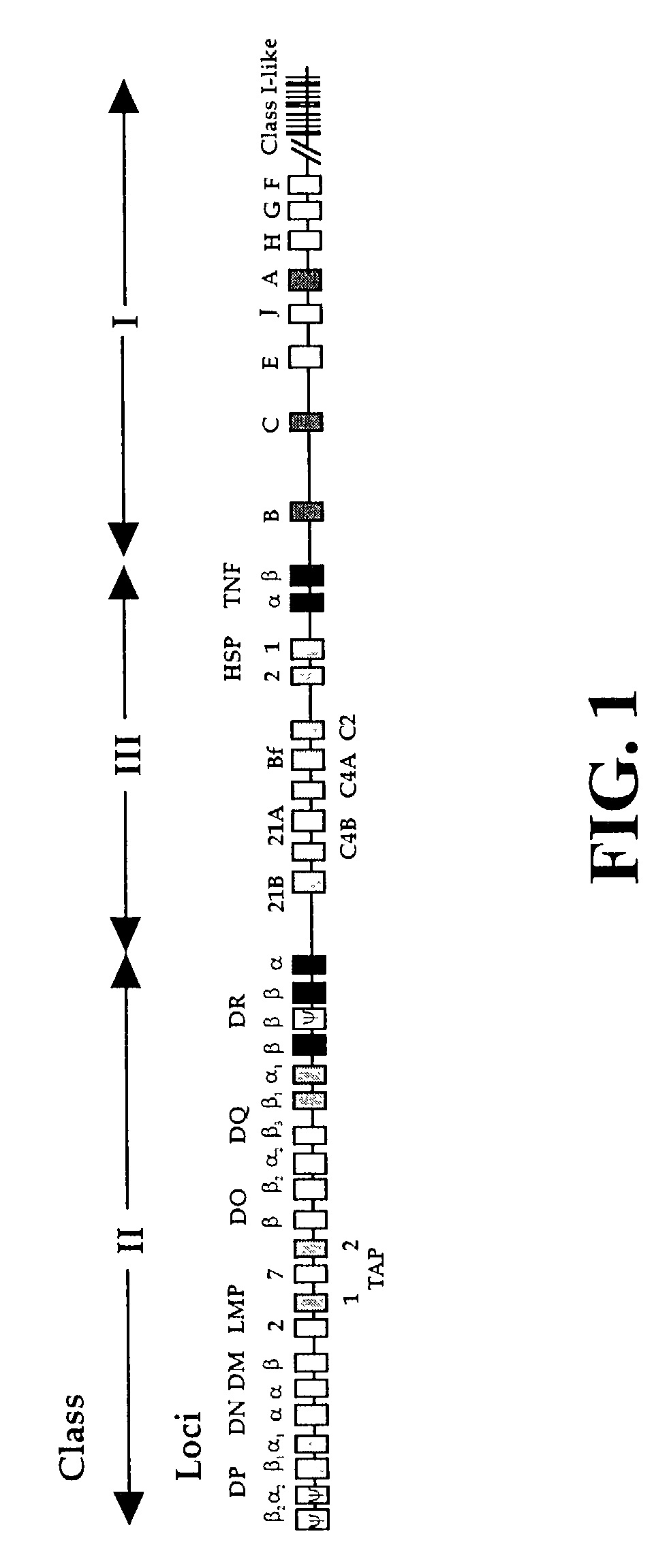
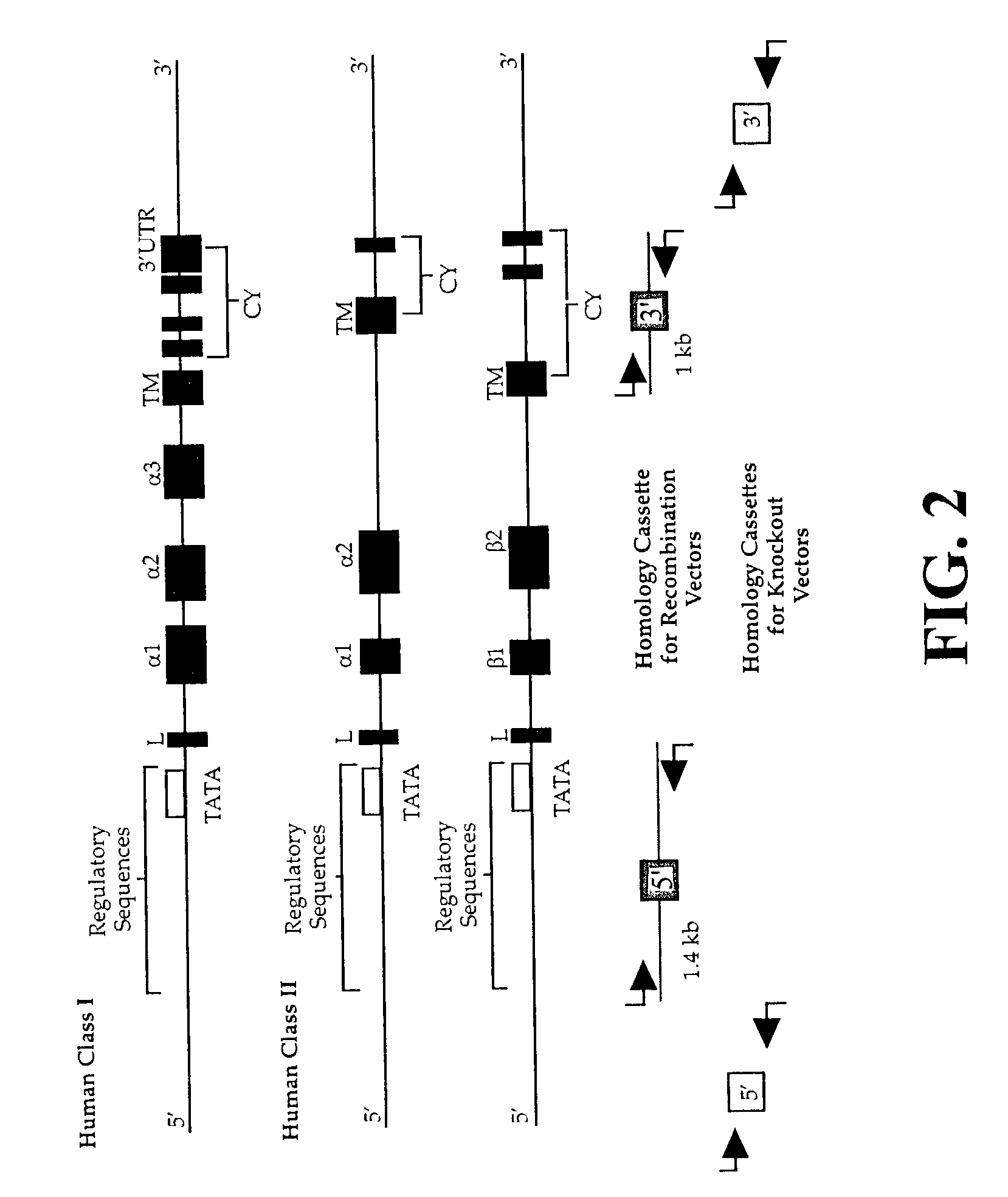
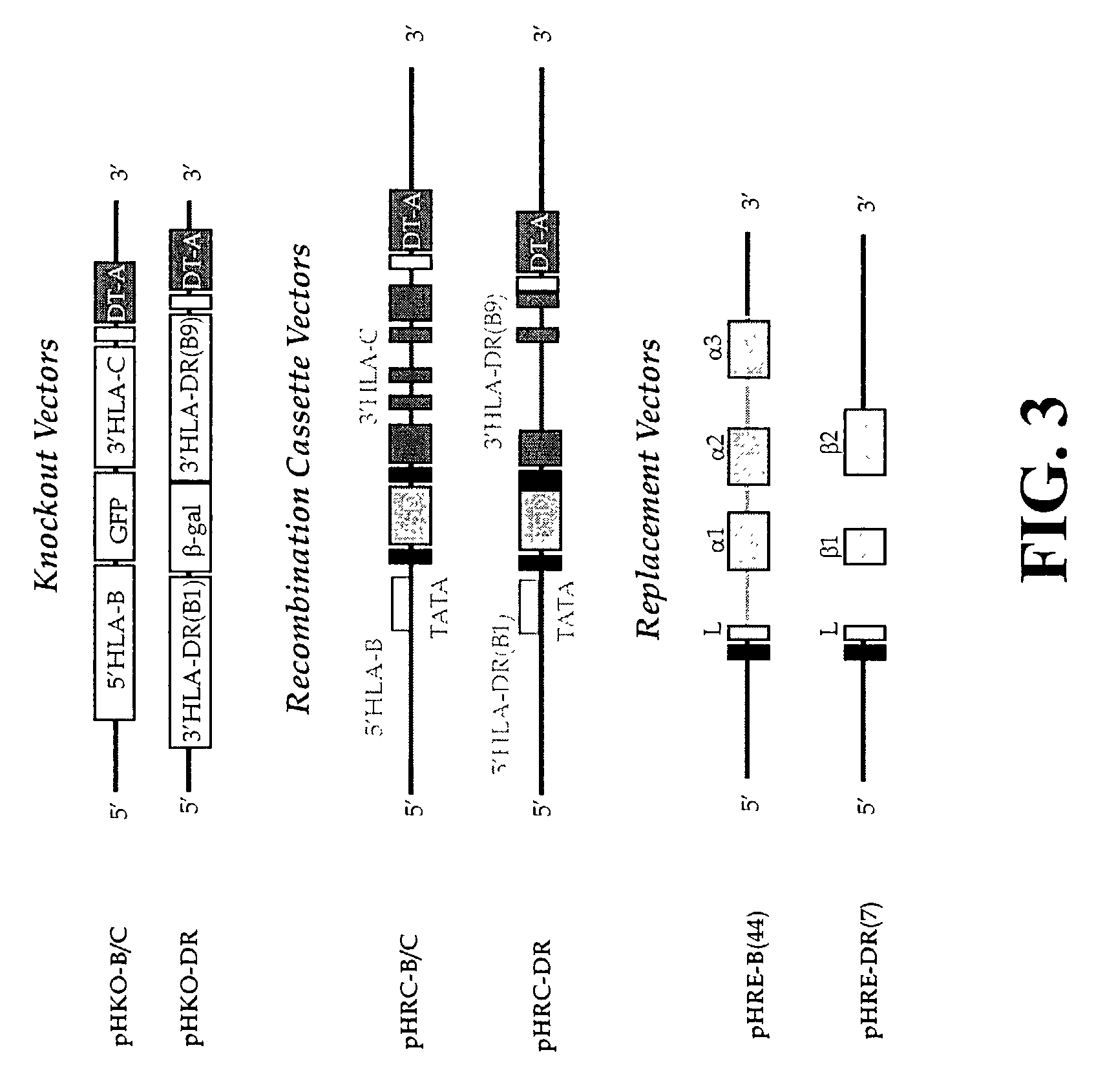
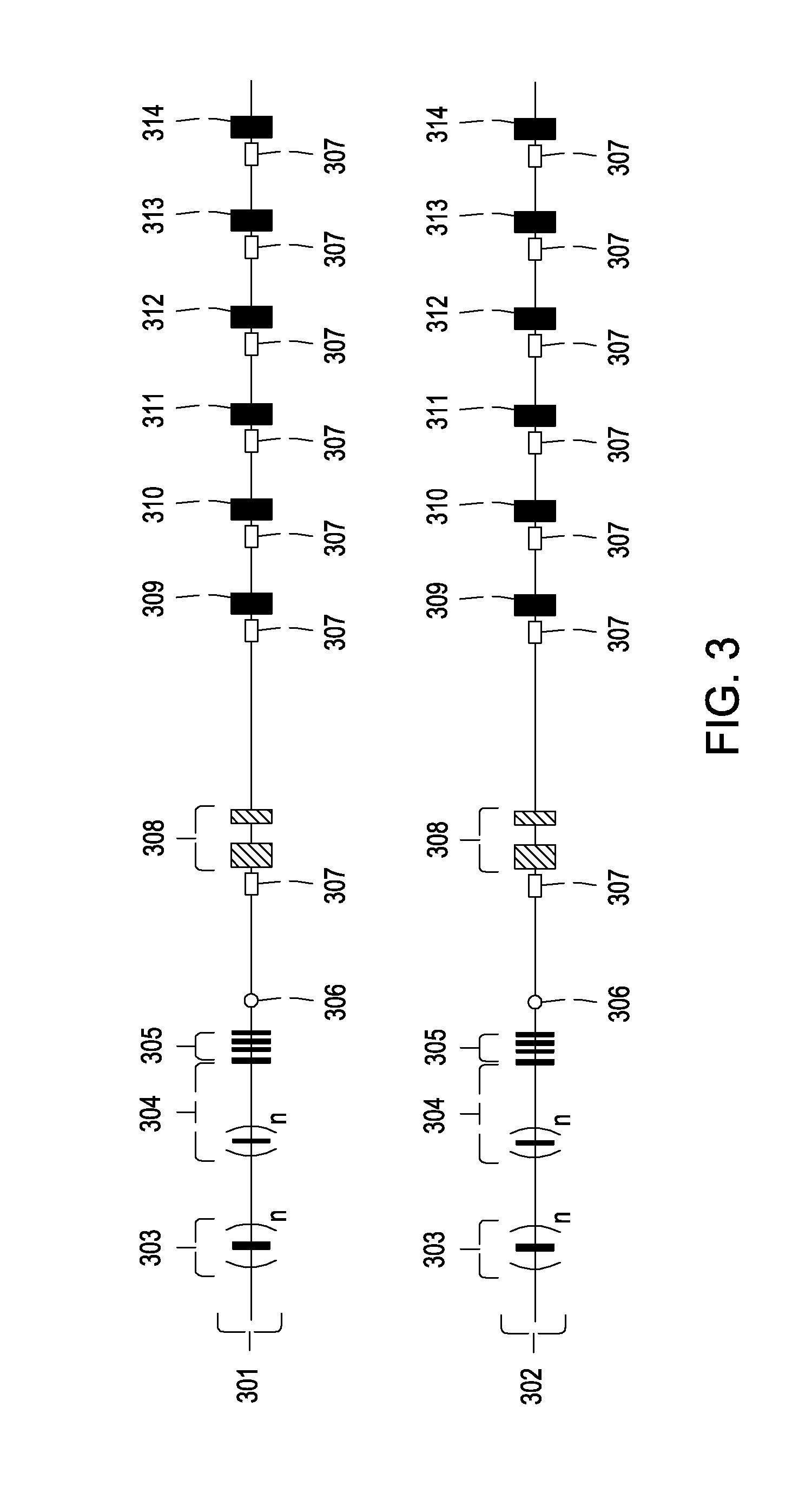
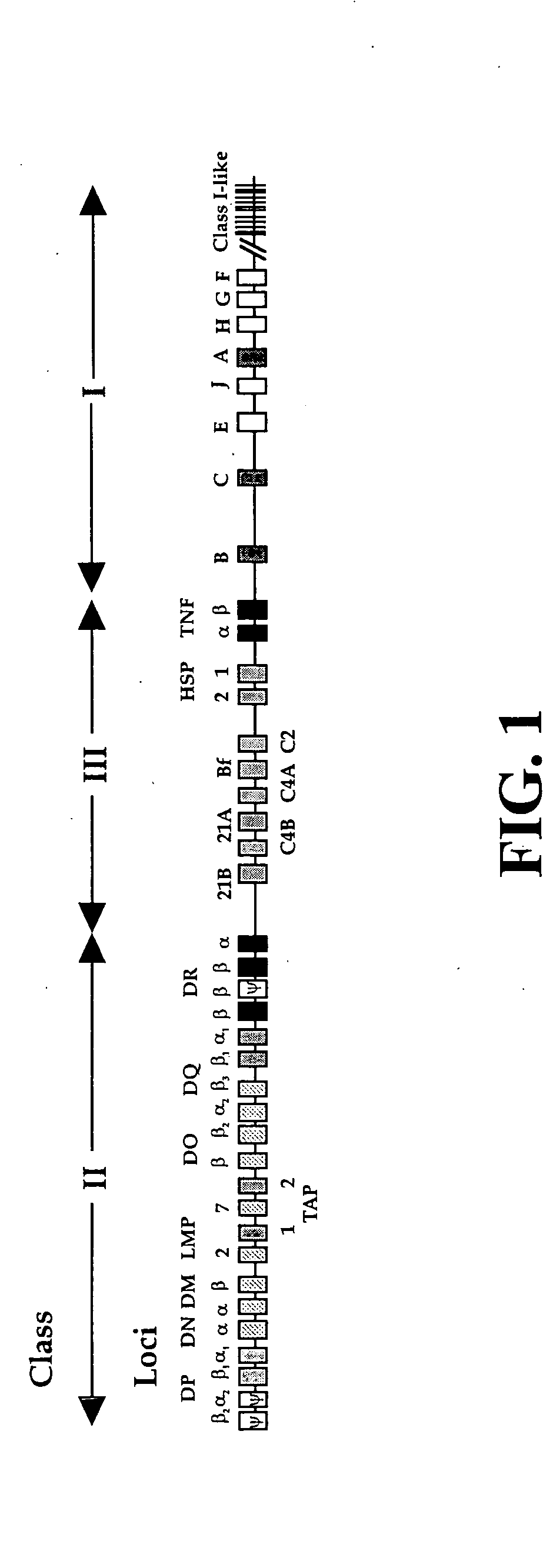
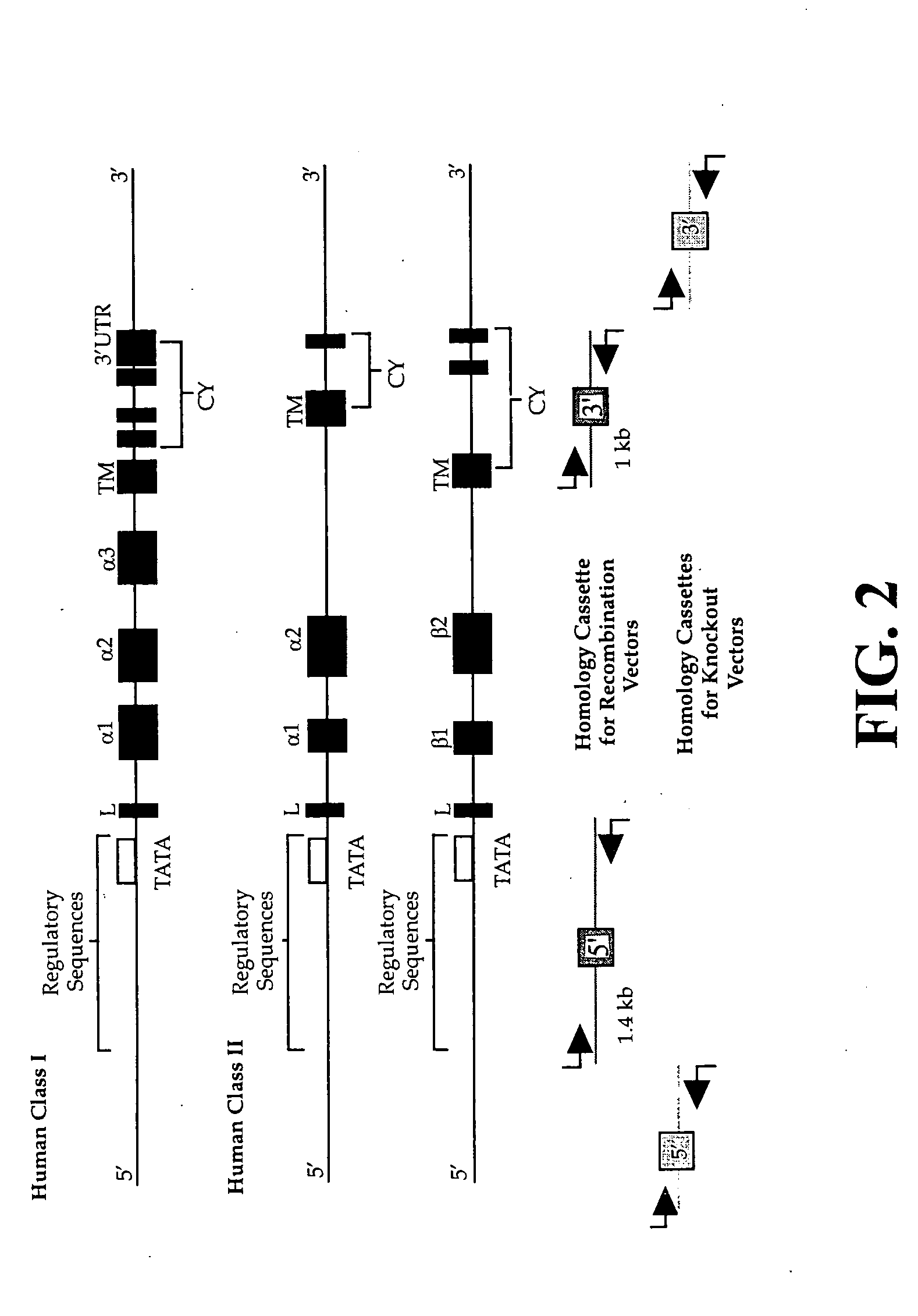
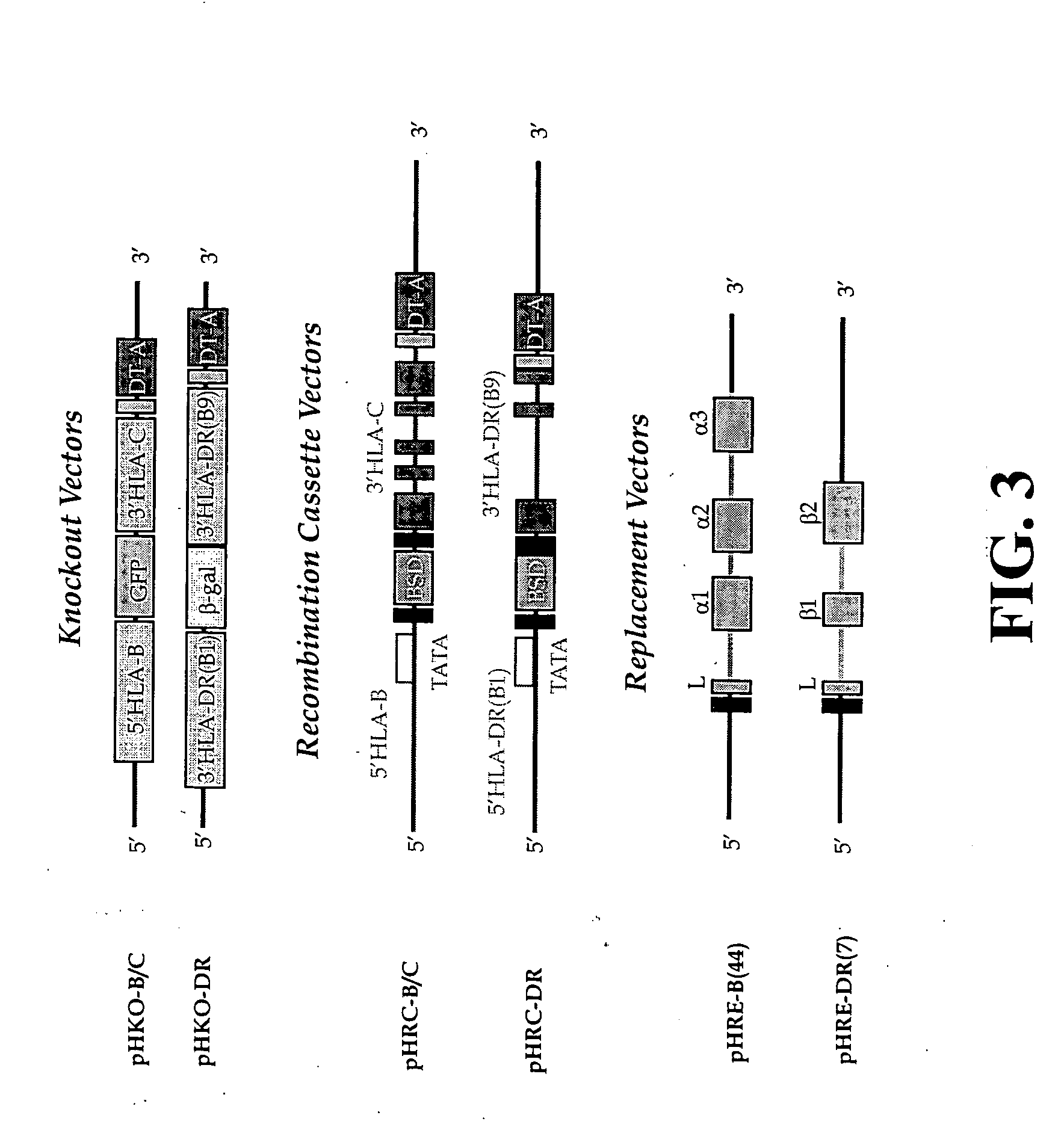
The subject invention pertains to materials and methods for preparing multi-potential stem cells having a pre-selected expression of MHC antigens. Stem cells of the subject invention can be used to generate histocompatible tissues / organs for transplantation. The process of the subject invention comprises the use of targeting vectors capable of gene knockout, insertion of site-specific recombination cassettes, and the replacement of histocompatibility alleles in the stem cell. Novel knockout vectors are used to delete designated regions of one chromosome. Recombination cassette vectors are then used to delete the same region on the second chromosome and deposit a site-specific recombination cassette which can be utilized by replacement vectors for inserting the new MHC genes on the chromosome of the engineered cell. The subject invention also pertains to cells, tissues, and transgenic mammal prepared using the methods and materials of the invention.
Owner:MORPHOGENESIS
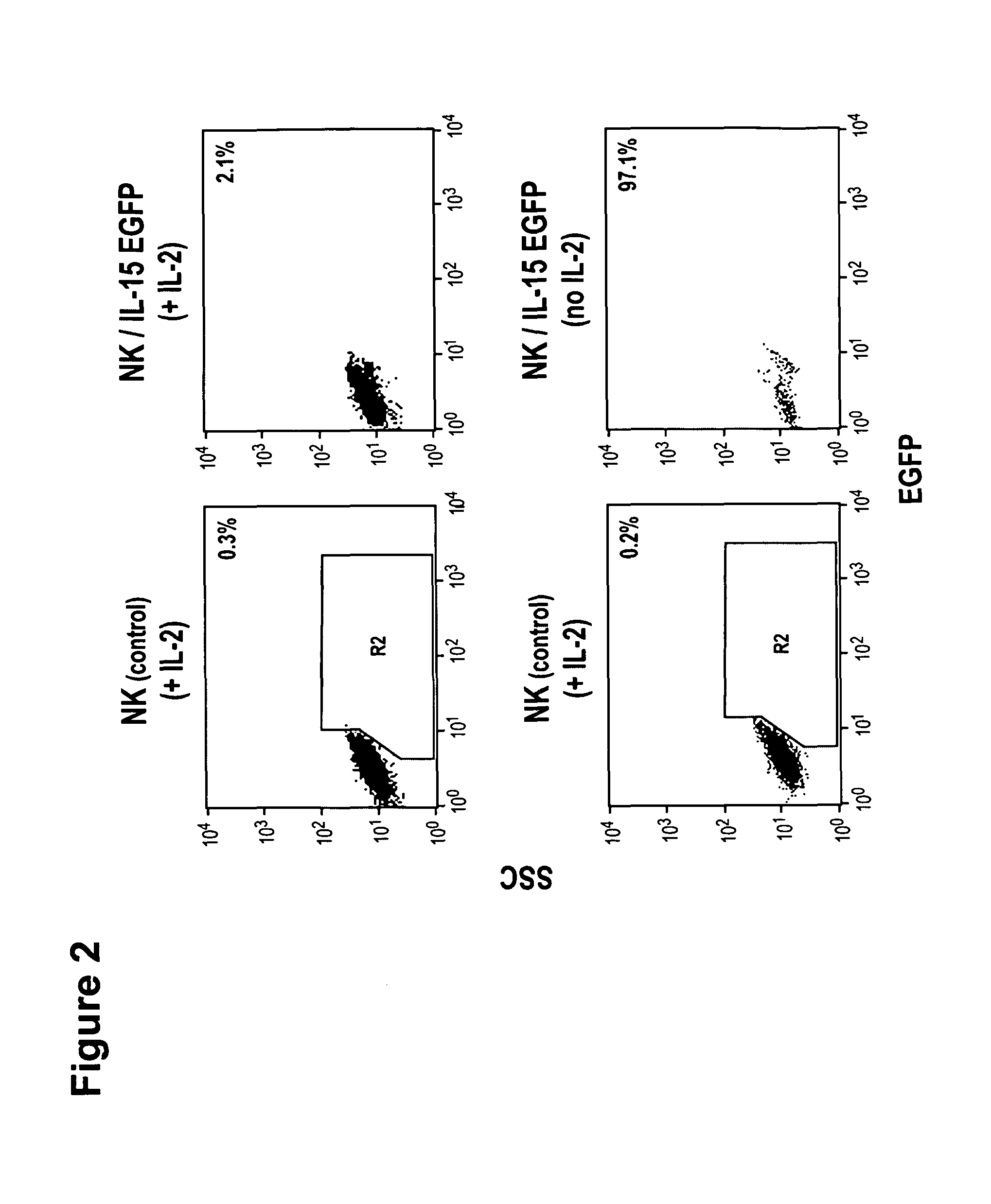
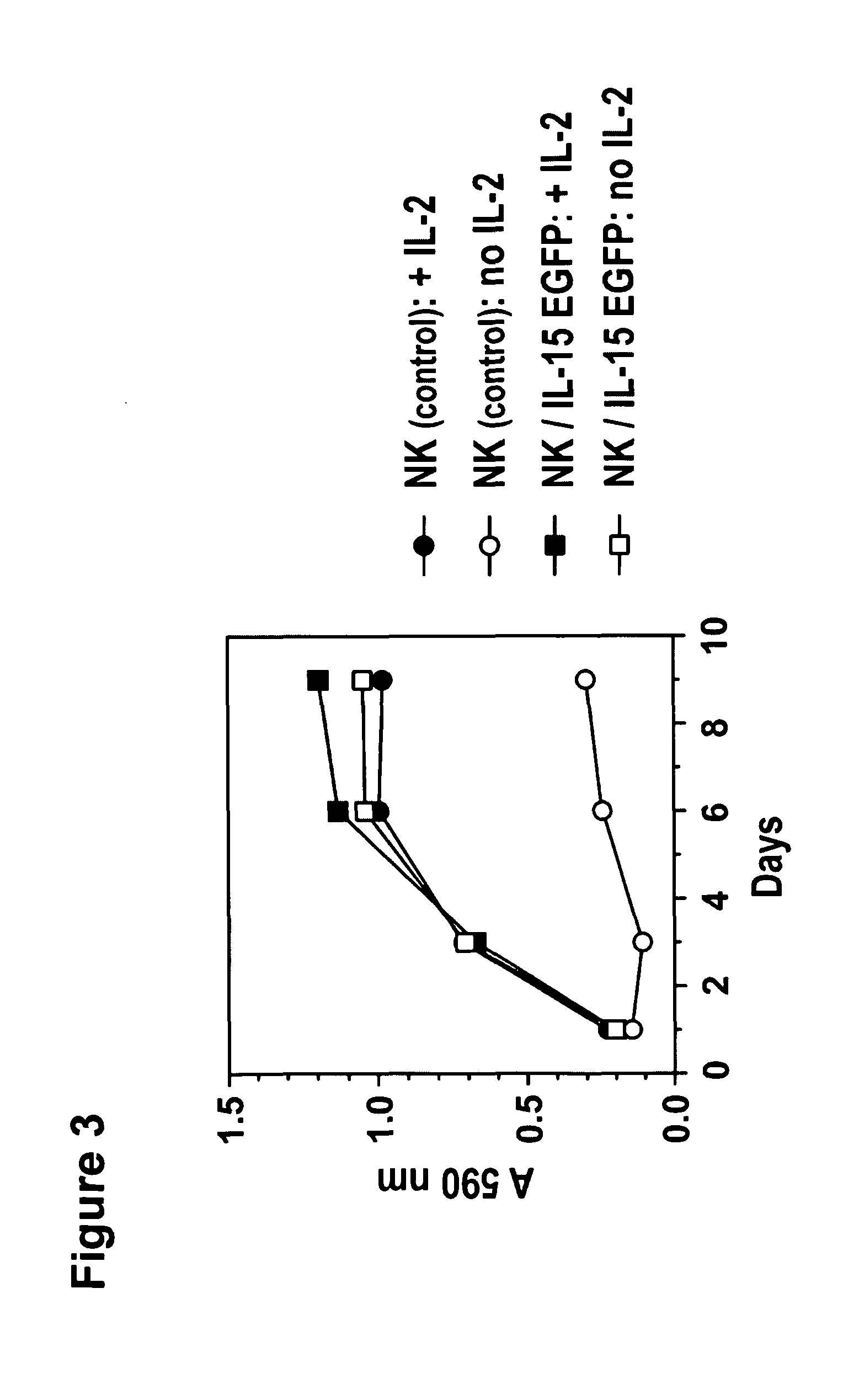
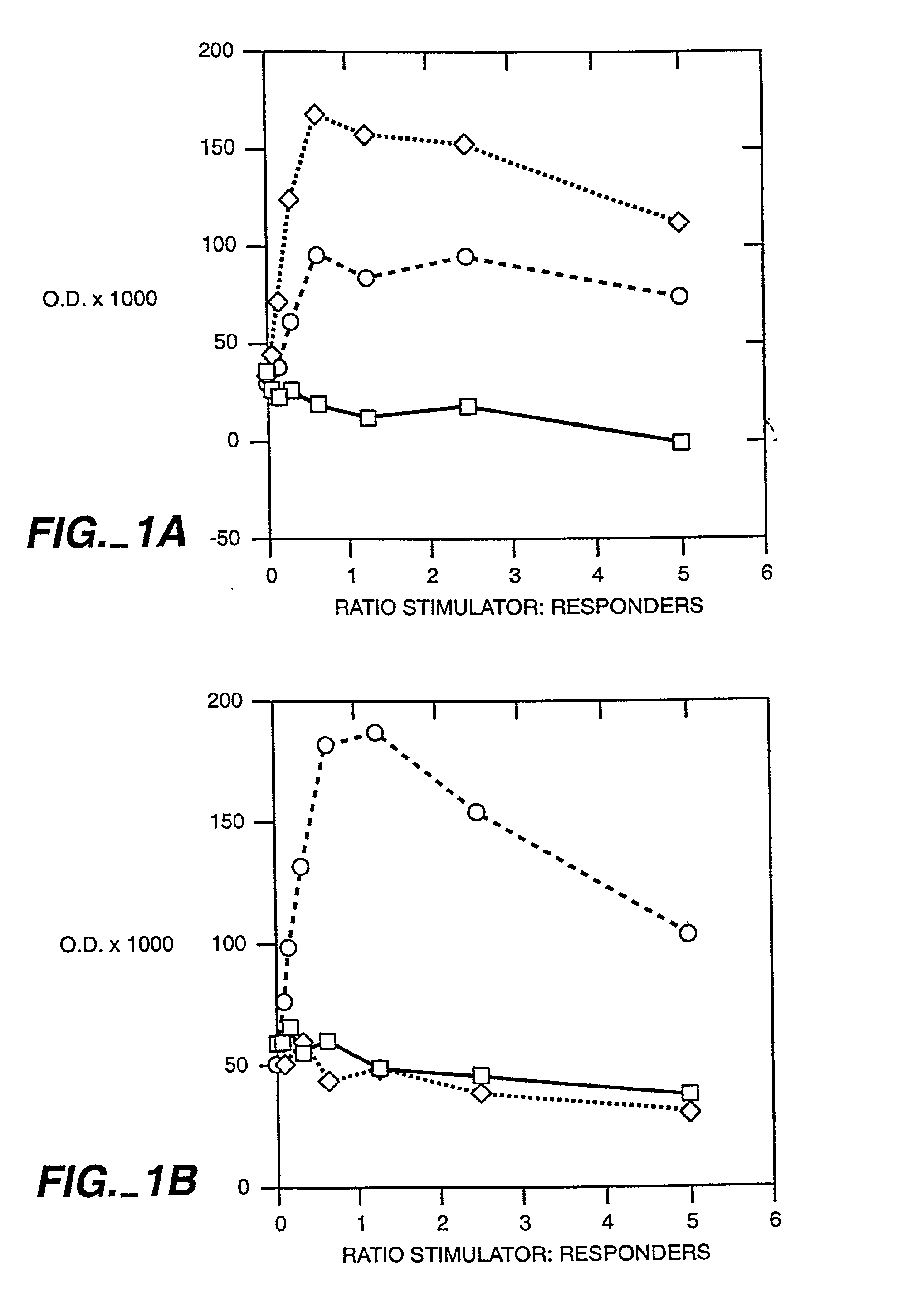
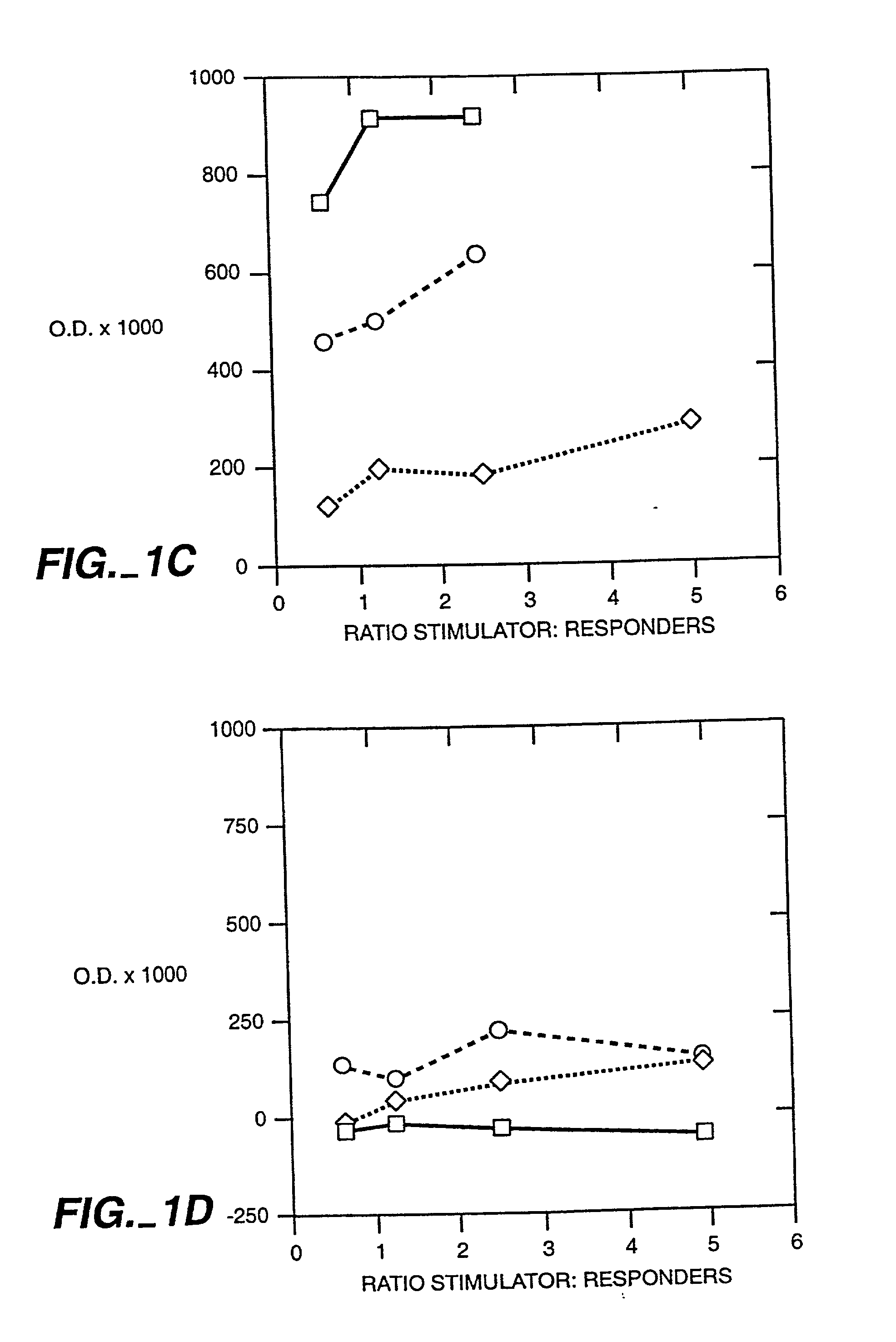
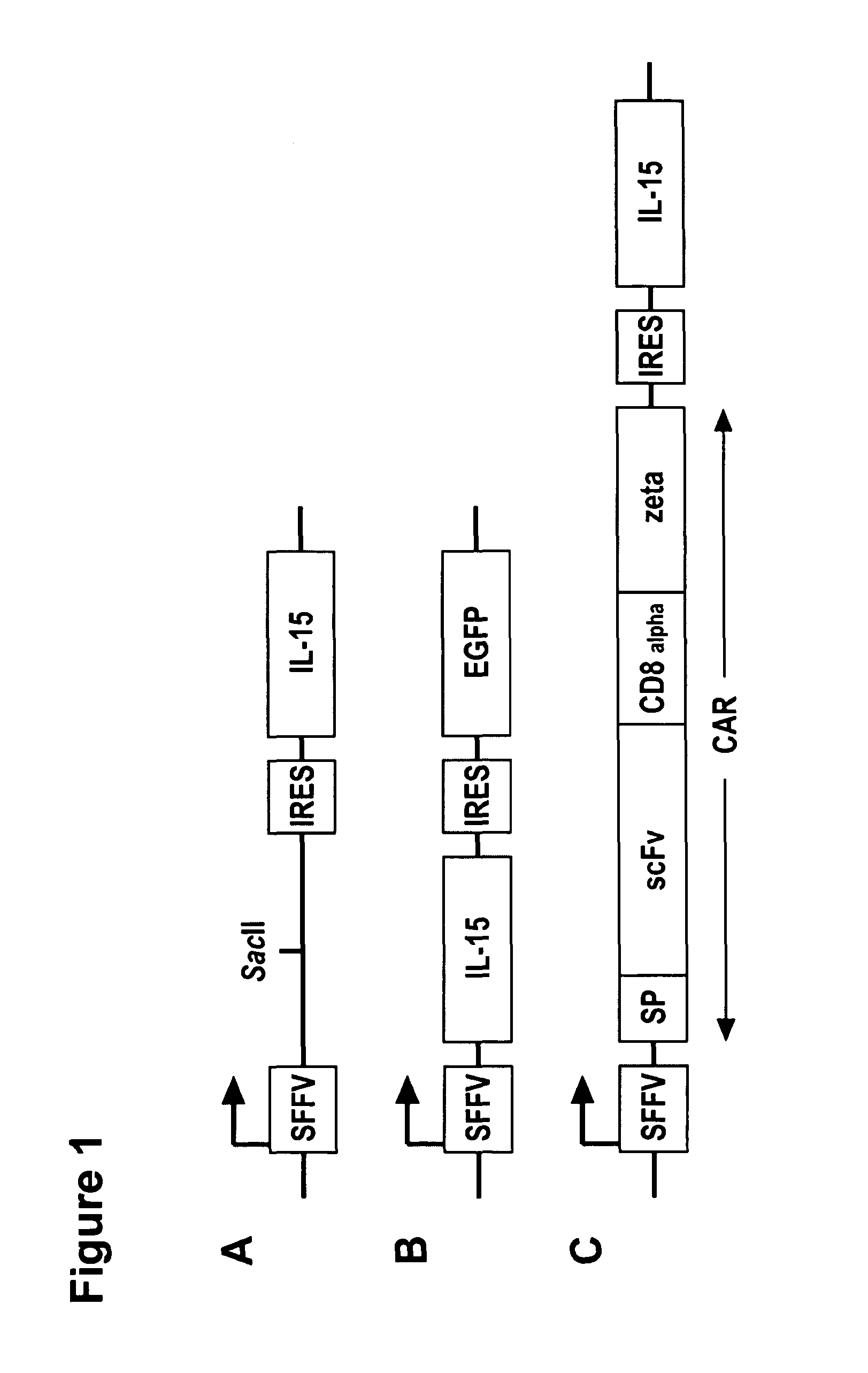
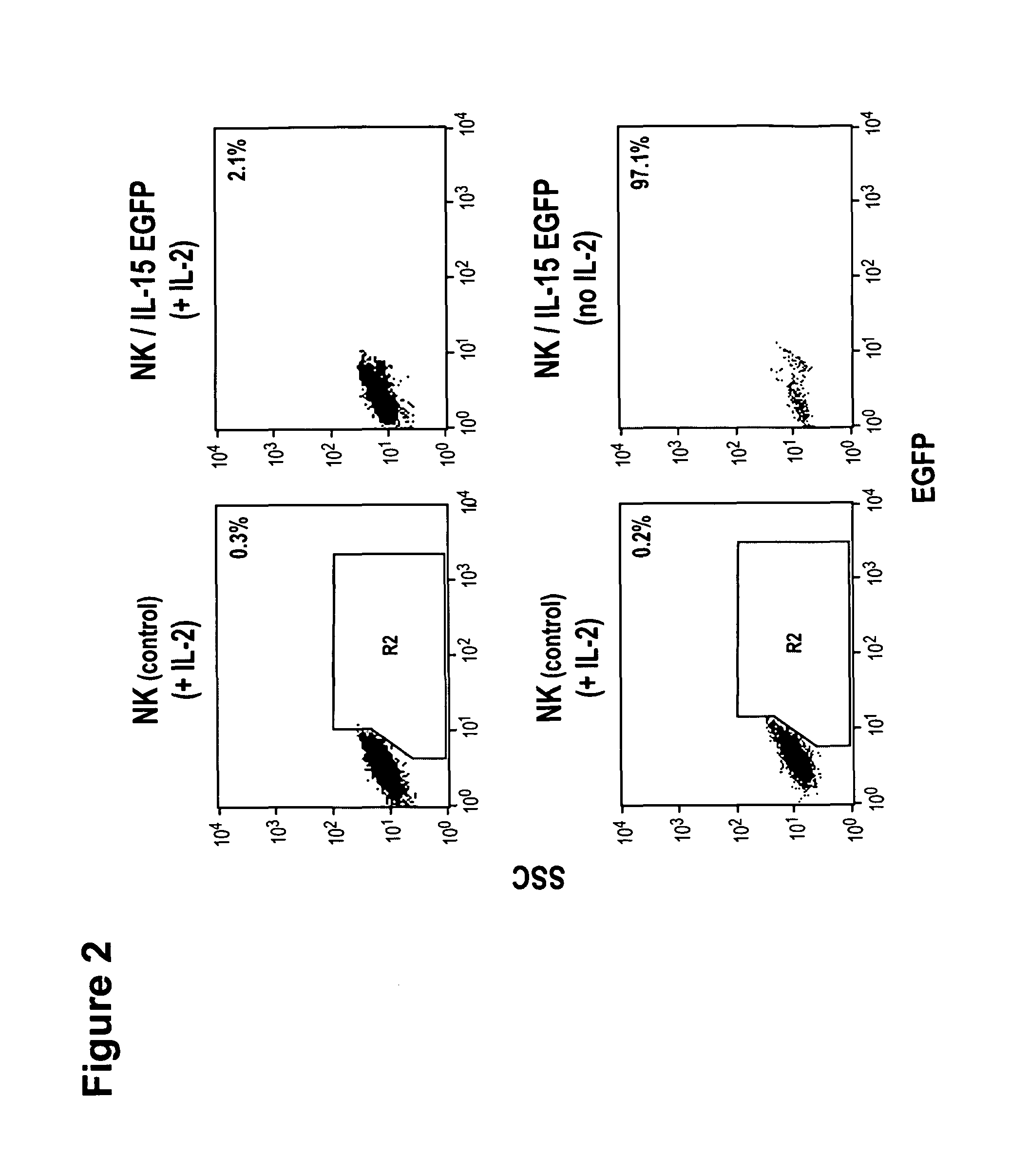
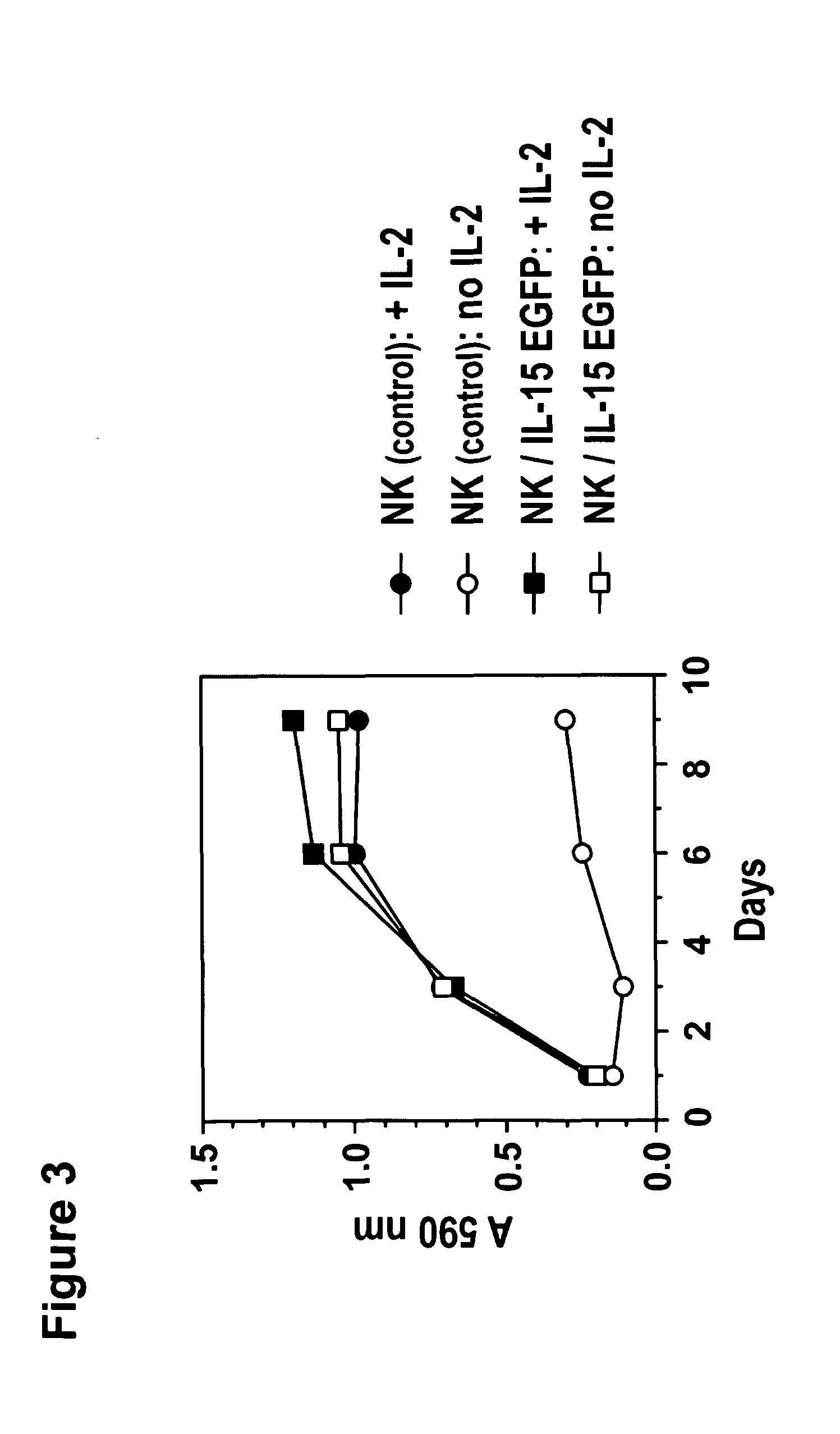
Interleukin 15 as Selectable Marker for Gene Transfer in Lymphocytes
ActiveUS20130280221A1Opening possibilityBiocideAntibody mimetics/scaffoldsADAMTS ProteinsAntigen receptors
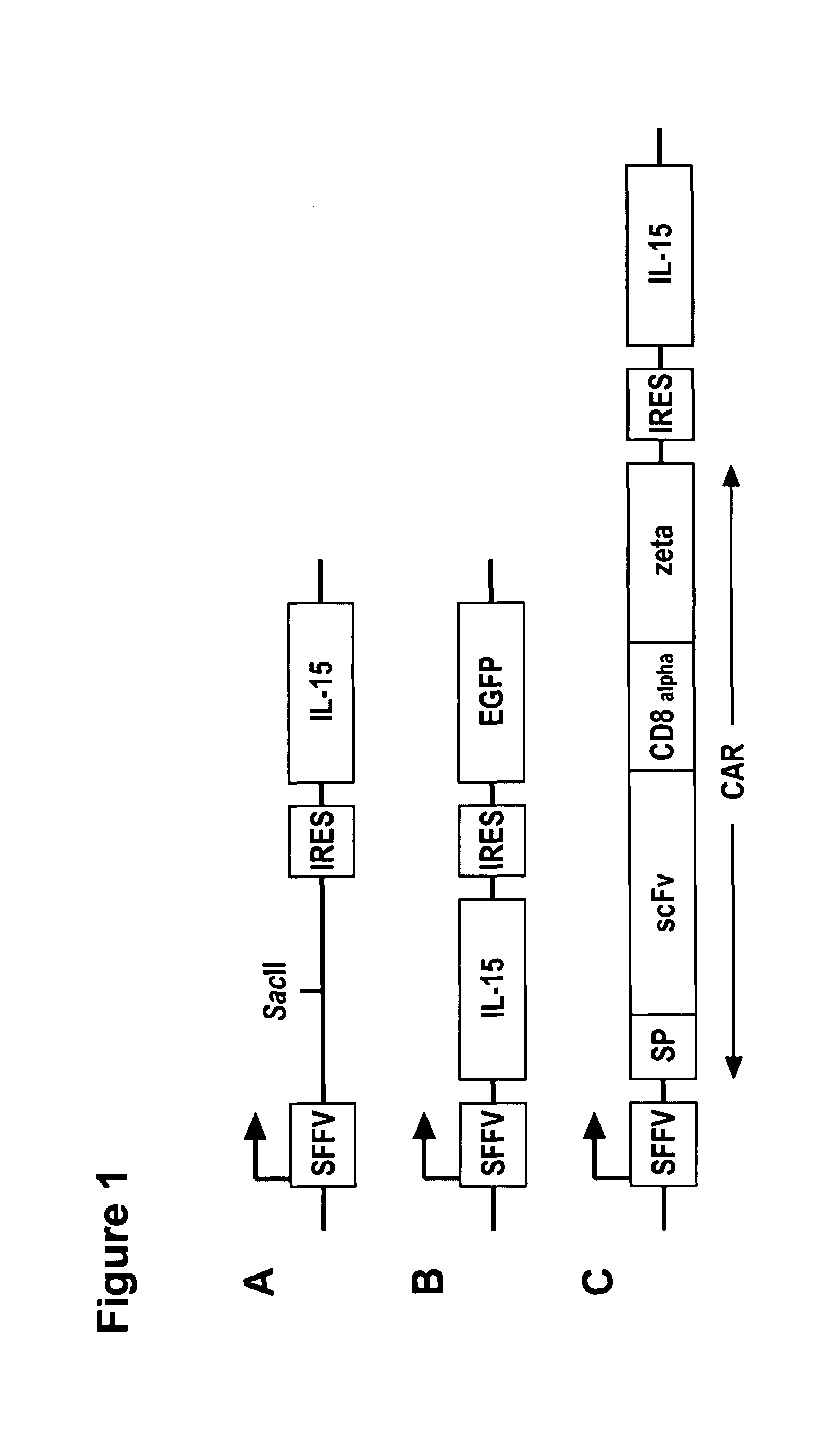
The present invention relates to the use of interleukin-15 (IL-15) as selectable marker for gene transfer, preferably of at least one gene of therapeutic interest, into a mammalian cell or cell line, in particular a human cell or cell line. The present invention furthermore relates to transgenic mammalian cells or cell lines expressing IL-15 as selectable marker and co-expressing at least one protein of interest encoded by at least one gene of interest, which is preferably a protein of therapeutic interest. The present invention is in particular suitable for chimeric antigen receptors (CARs) as the gene or protein of interest and their expression in lymphocytes. The transgenic mammalian cells and cell lines are furthermore suitable for use as a medicament, in particular in the treatment of cancer and in immunotherapy, such as adoptive, target-cell specific immunotherapy.
Owner:CHEMOTHERAPEUTISCHES FORSCHUNGINSTITUT GEORG SPEYER HAUS
Transgenic mammal capable of facilitating production of donor-specific functional immunity
This invention provides for transgenic non-human mammalian models of human disease, methods of making such models as well as methods of using such models to assess efficacy of therapeutic and prophylaxis treatments, to assess the antigenic potential of compounds, and other uses.
Owner:GENENCOR INT INC
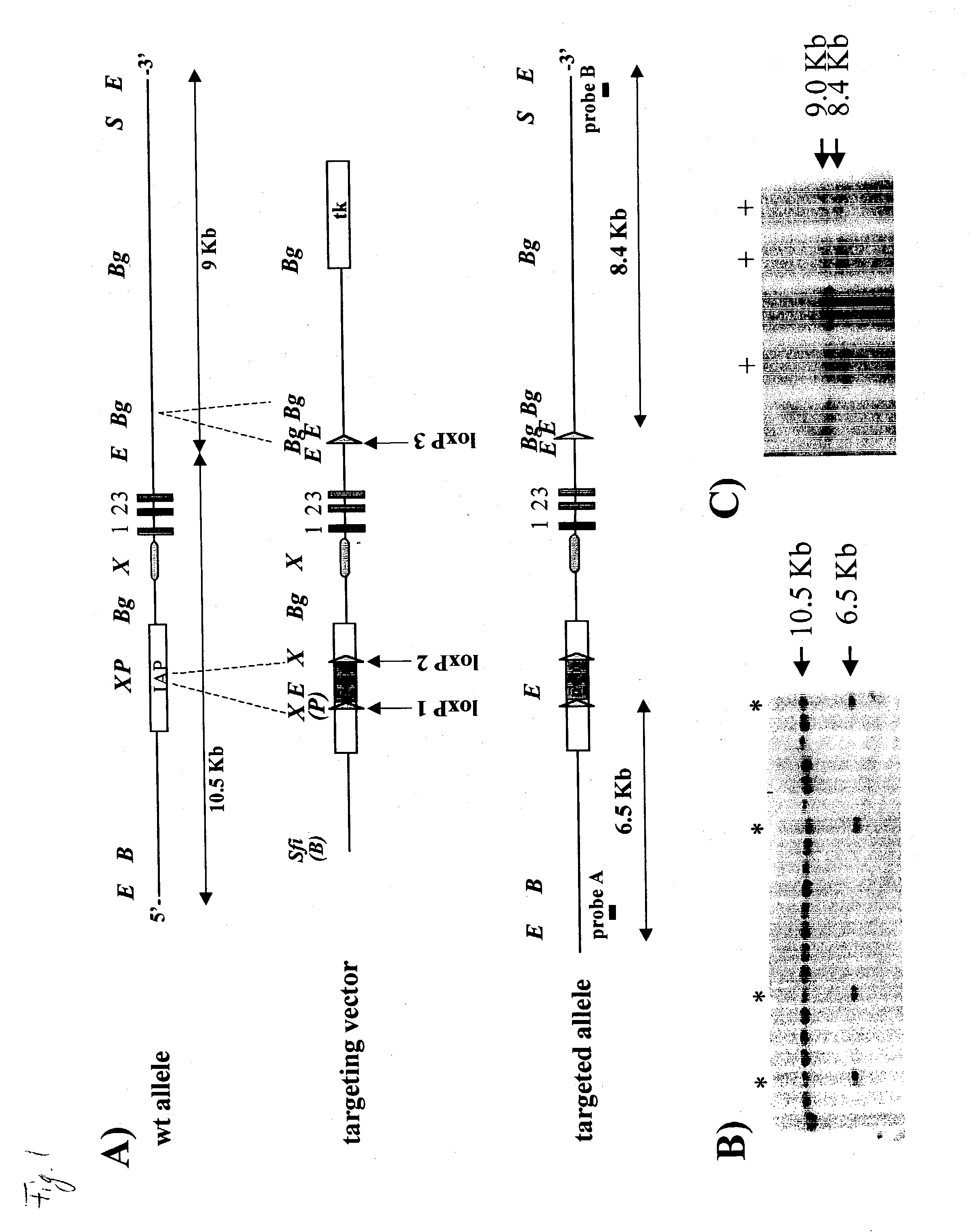
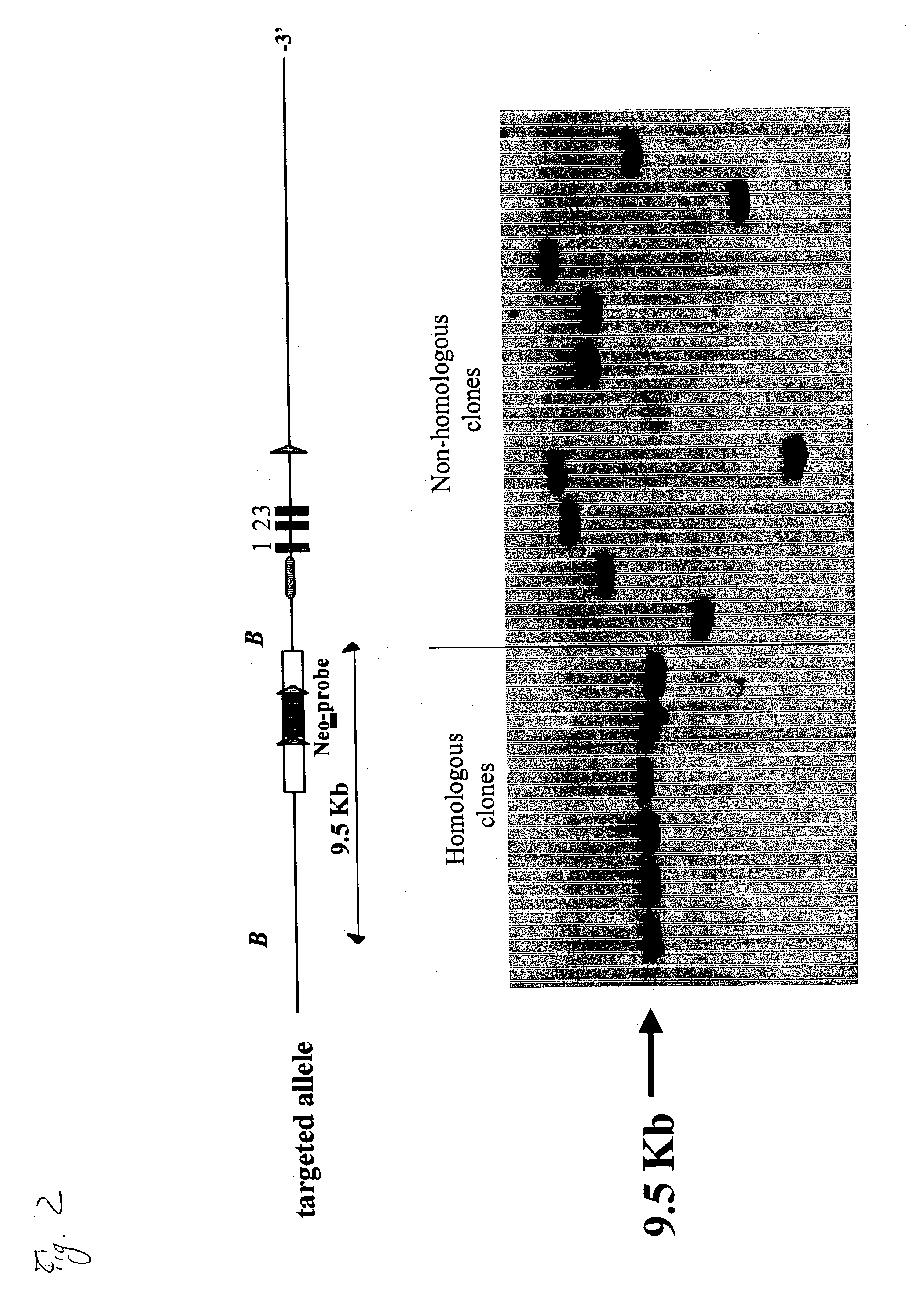
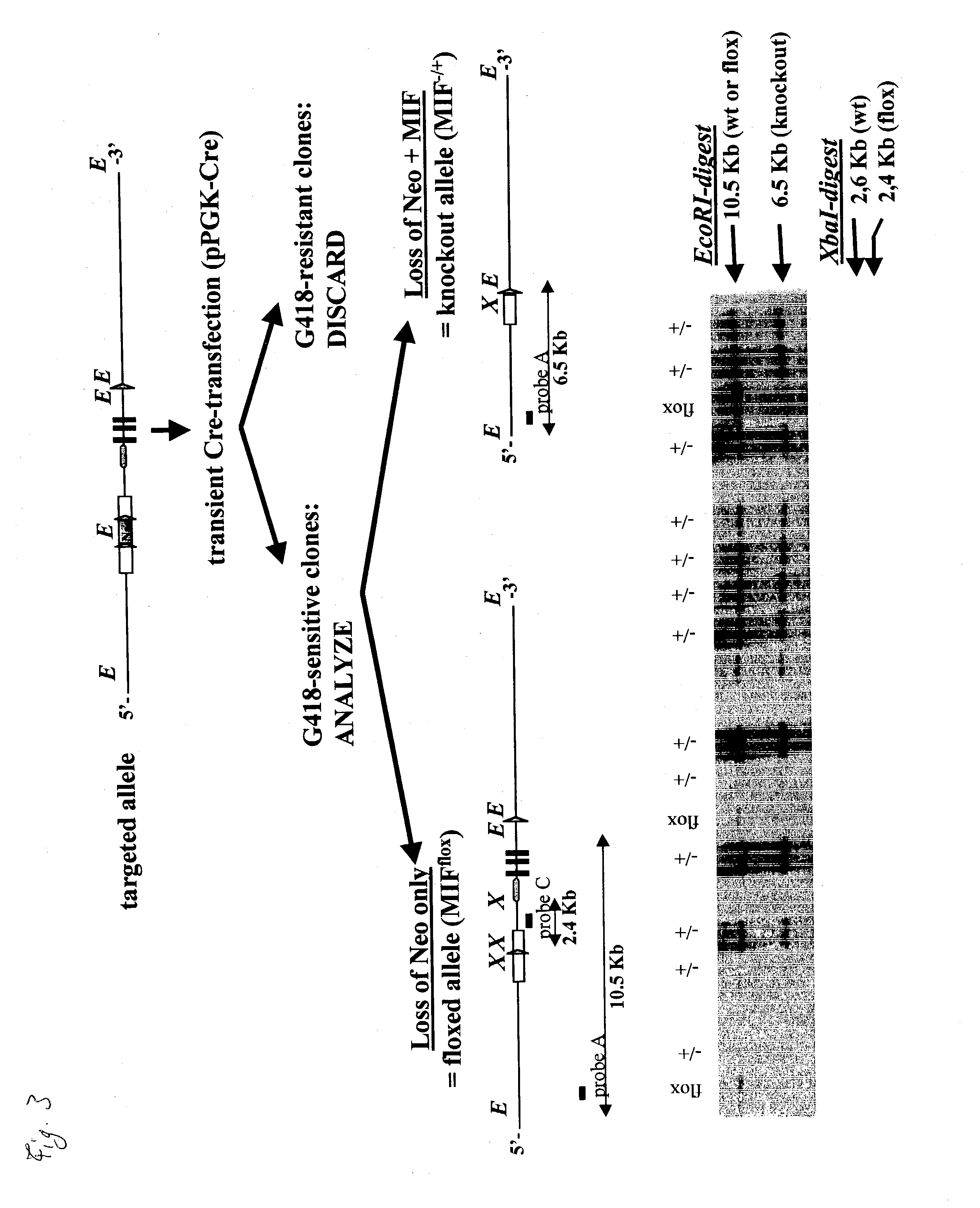
Non-human mammal with disrupted or modified MIF gene, and uses thereof
The present invention demonstrates transgenic mammals, particularly transgenic mice, having a genomic disruption or mutation affecting the MIF gene. The invention is also directed to use of the transgenic mice in developing therapies to inflammatory or neoplastic disorders involving MIF cellular activity.
Owner:FINGERLE ROWSON GUNTER R +1
Methods for producing sialyloligosaccharides in a dairy source
InactiveUS6323008B1High yieldBroaden the fieldVectorsSugar derivativesHigh concentrationEnzymatic synthesis
The present invention provides methods for producing sialyloligosaccharides in situ in dairy sources and cheese processing waste streams, prior to, during, or after processing of the dairy source during the cheese manufacturing process. The methods of the present invention use the catalytic activity of alpha(2-3) trans-sialidases to exploit the high concentrations of lactose and alpha(2-3) sialosides which naturally occur in dairy sources and cheese processing waste streams to drive the enzymatic synthesis of alpha(2-3) sialyllactose. alpha(2-3) sialyloligosaccharides produced according to these methods are additionally encompassed by the present invention. The invention also provides for recovery of the sialyloligosaccharides produced by these methods. The invention further provides a method for producing alpha(2-3) sialyllactose. The invention additionally provides a method of enriching for alpha(2-3) sialyllactose in milk using transgenic mammals that express an alpha(2-3) trans-sialidase transgene. The invention also provides for recovery of the sialyllactose contained in the milk produced by this transgenic mammal either before or after processing of the milk. Transgenic mammals containing an alpha(2-3) trans-sialidase encoding sequence operably linked to a regulatory sequence of a gene expressed in mammary tissue are also provided by the invention.
Owner:NEOSE TECH
PRODUCTION OF BONE MORPHOGENIC PROTEINS (BMPs) IN TRANSGENIC MAMMALS
The present invention provides materials and methods for the production of recombinant BMPs in transgenic animals. In particular, the invention provides materials and methods for the production of recombinant BMPs in the milk of transgenic animals that express recombinant BMPs in the mammary gland.
Owner:CLOKIE CAMERON MALCOLM LANG +2
Method for the production of fusion proteins in transgenic mammal milk
InactiveUS20060105347A1Short timeEfficient productionFungiPeptide/protein ingredientsHalf-lifeBiocompatibility Testing
Desirable fusion proteins can be produced in and purified from the milk of transgenic animals. The peptides are made as fusion proteins with a suitable fusion partner such as human alpha-fetoprotein. The fusion partner protein acts to promote and increase the half-life of the overall molecule as well as having therapeutic effects on its own. The fusion protein is typically produced through the use of transgenic animals and can be purified away from the now the milk or other bodily fluid of such an animal by an affinity purification method. A particular advantage of producing peptides via this route, in addition to the obvious advantages of high yield and biocompatibility, is that specific post-translational modifications, such as carboxy terminal amidation, can be performed in the mammary gland. Biologically active polypeptides comprising a therapeutically active polypeptide fused to human alpha-fetoprotein fragment or a variant thereof, methods for the preparation thereof, nucleotide sequences encoding such fusion polypeptides, expression cassettes comprising such nucleotide sequences, self-replicating plasmids containing such expression cassettes, and pharmaceutical compositions containing said fusion polypeptides.
Owner:GTC BIOTHERAPEUTICS INC
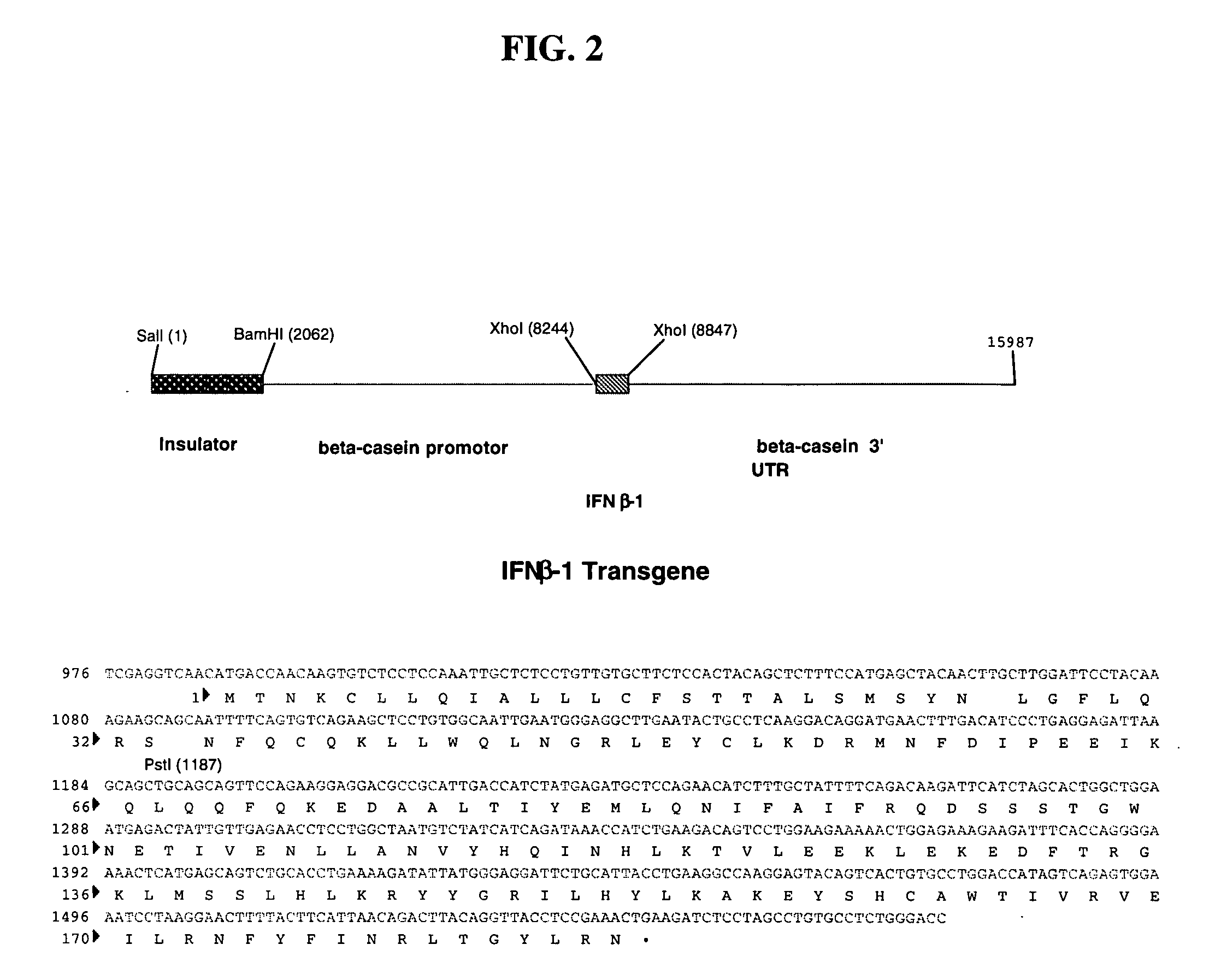
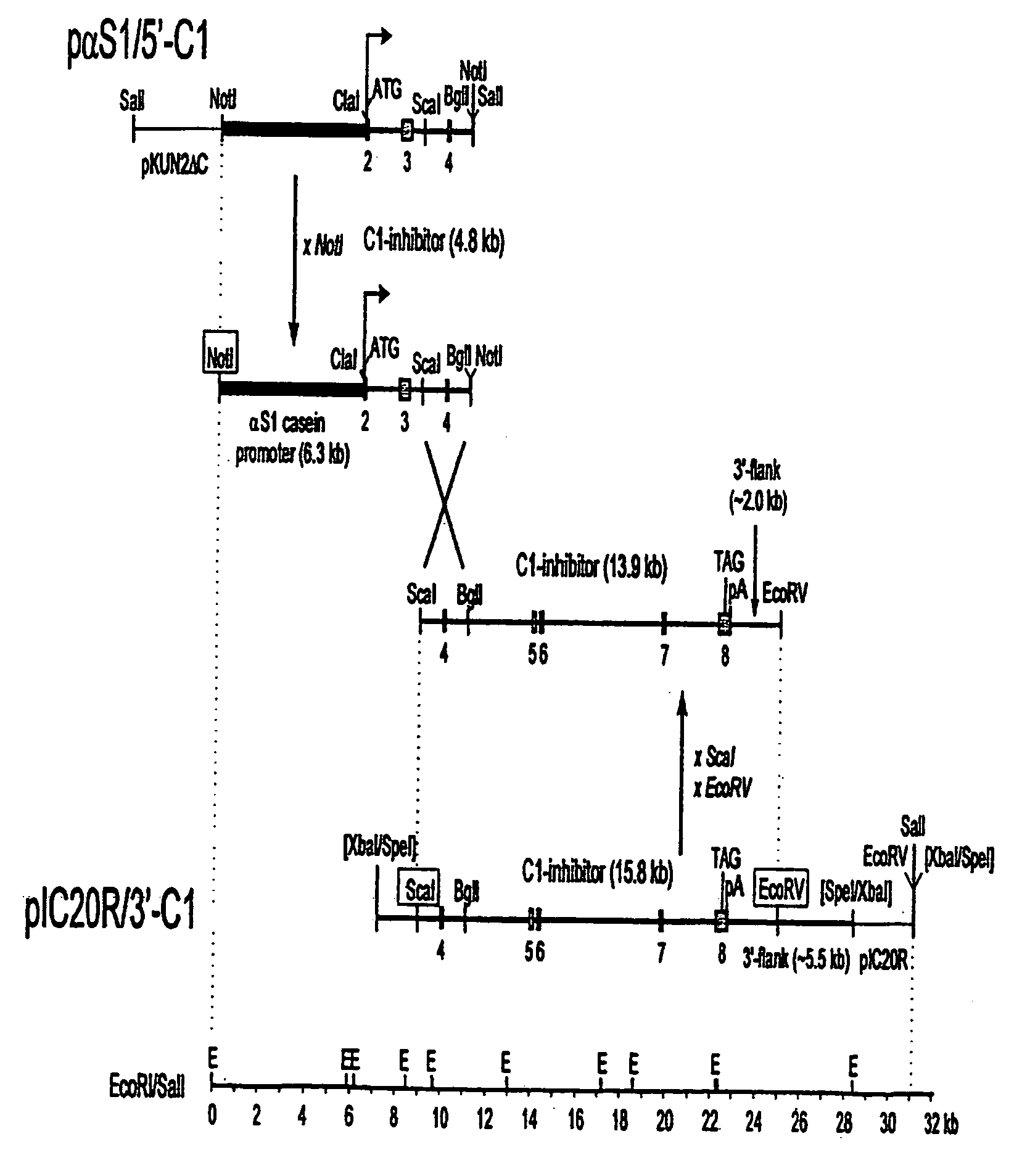
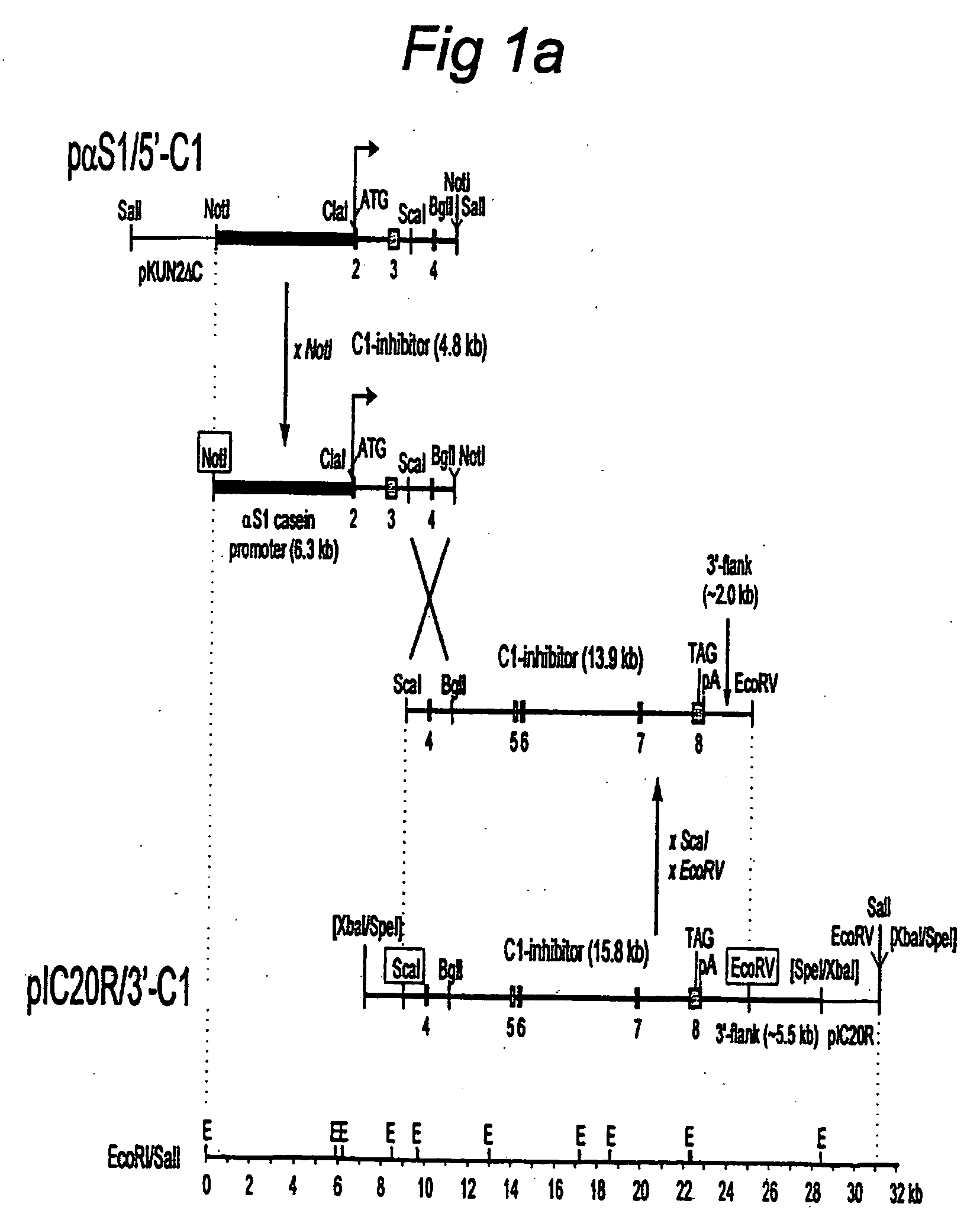
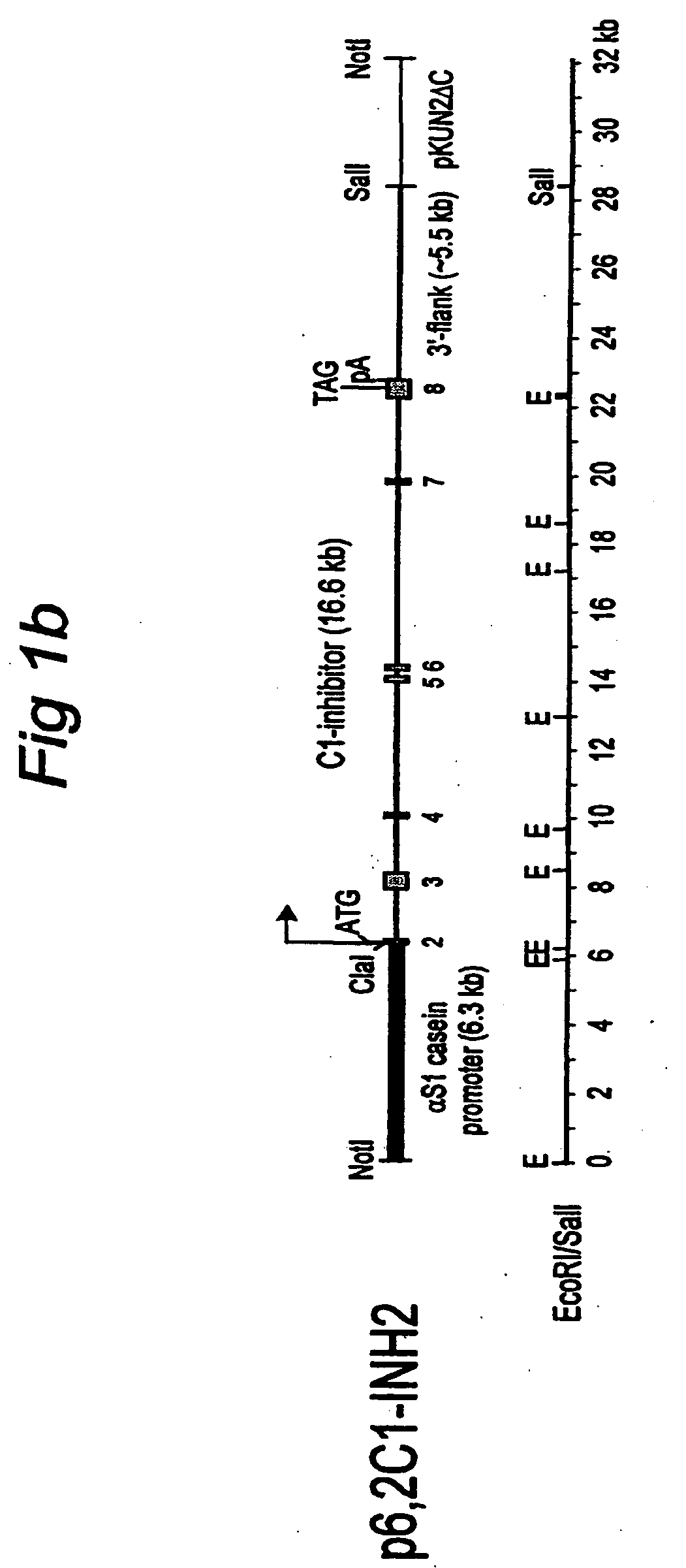
C1 inhibitor produced in the milk of transgenic mammals
The invention provides transgenic nonhuman mammals expressing C1 inhibitor in their milk. The C1 inhibitor is useful in treating patients with hereditary angioedema or patients requiring immunosuppression.
Owner:PHARMING INTPROP BV
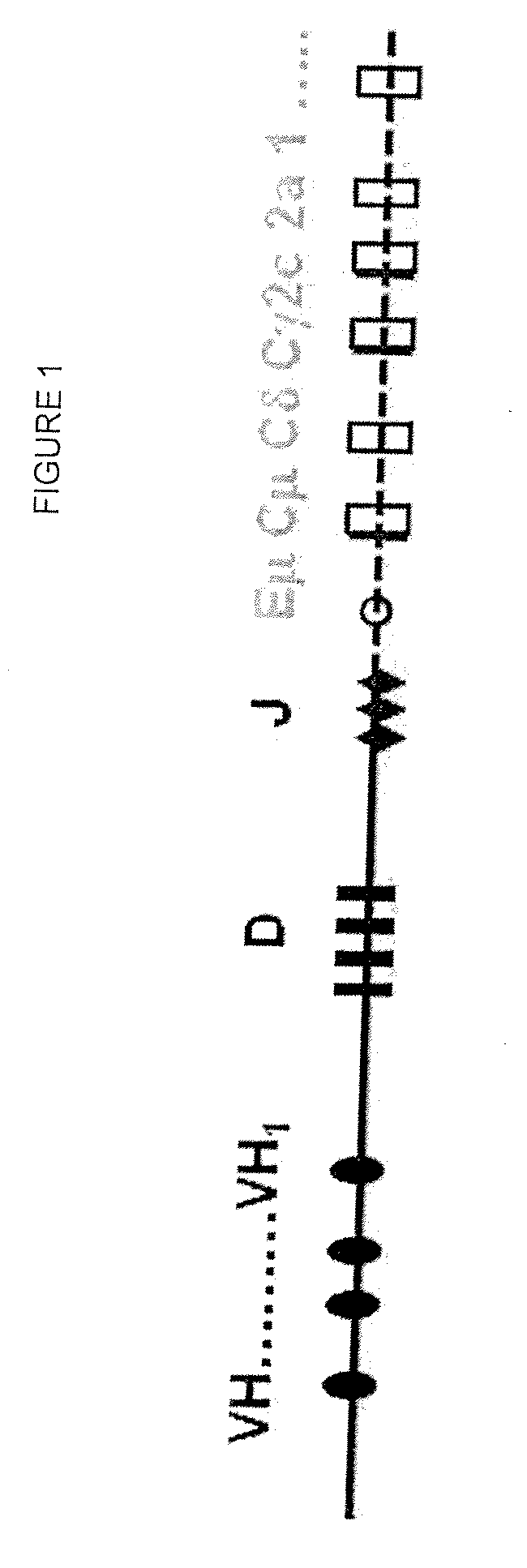
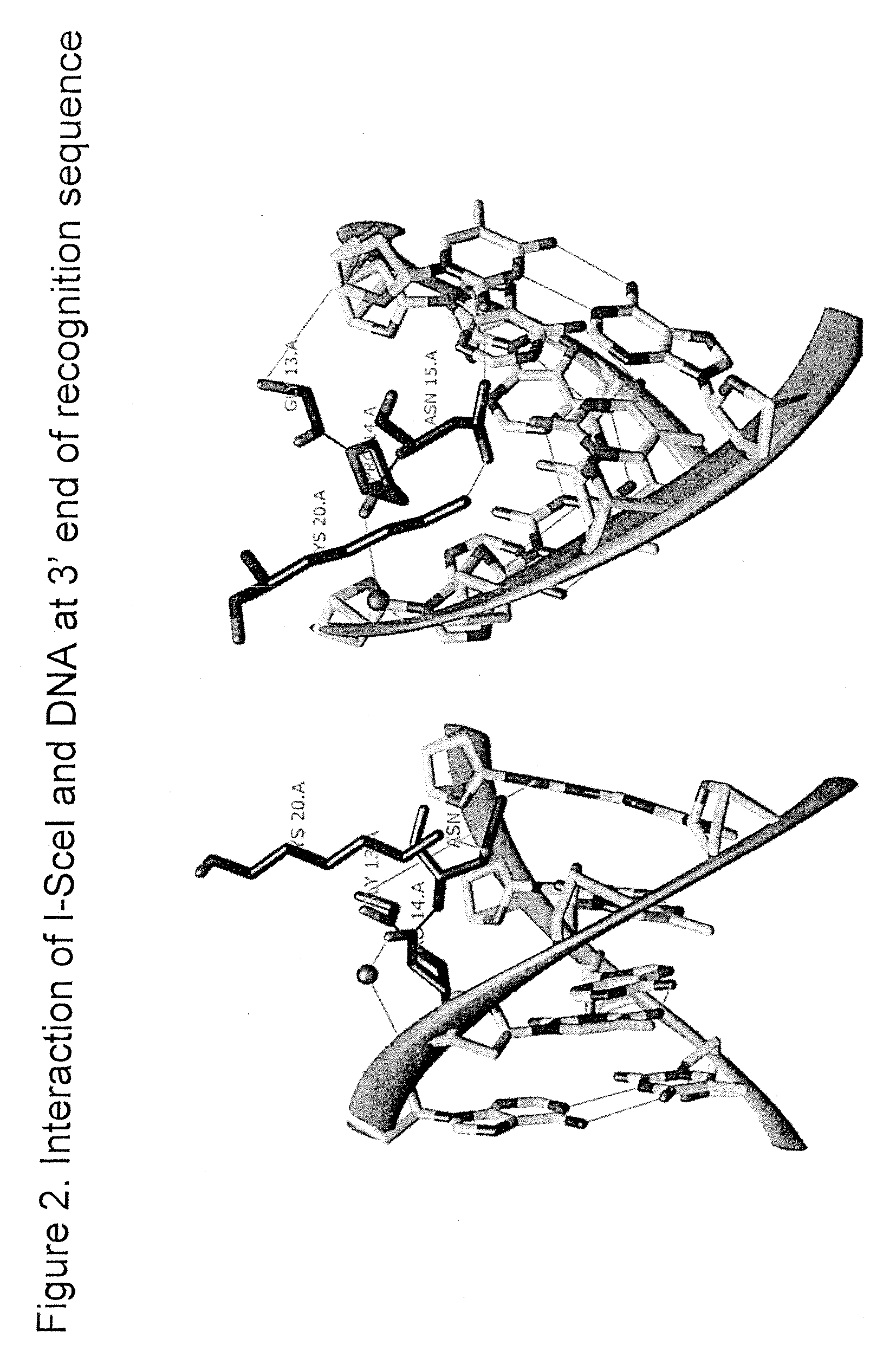
Compositions and Methods for Producing Transgenic Mammals Having Recombinant Immunoglobulin Loci
The invention provides methods for the production of transgenic animals comprising a recombinant Ig locus, as well as transgenic antibodies derived therefrom. The methods involve meganuclease cleavage-stimulated homologous recombination in mammalian embryos.
Owner:OMNIAB OPERATIONS INC
Interleukin 15 as selectable marker for gene transfer in lymphocytes
ActiveUS9487800B2Opening possibilityBiocideAntibody mimetics/scaffoldsADAMTS ProteinsAntigen receptors
The present invention relates to the use of interleukin-15 (IL-15) as selectable marker for gene transfer, preferably of at least one gene of therapeutic interest, into a mammalian cell or cell line, in particular a human cell or cell line. The present invention furthermore relates to transgenic mammalian cells or cell lines expressing IL-15 as selectable marker and co-expressing at least one protein of interest encoded by at least one gene of interest, which is preferably a protein of therapeutic interest. The present invention is in particular suitable for chimeric antigen receptors (CARs) as the gene or protein of interest and their expression in lymphocytes. The transgenic mammalian cells and cell lines are furthermore suitable for use as a medicament, in particular in the treatment of cancer and in immunotherapy, such as adoptive, target-cell specific immunotherapy.
Owner:CHEMOTHERAPEUTISCHES FORSCHUNGINSTITUT GEORG SPEYER HAUS
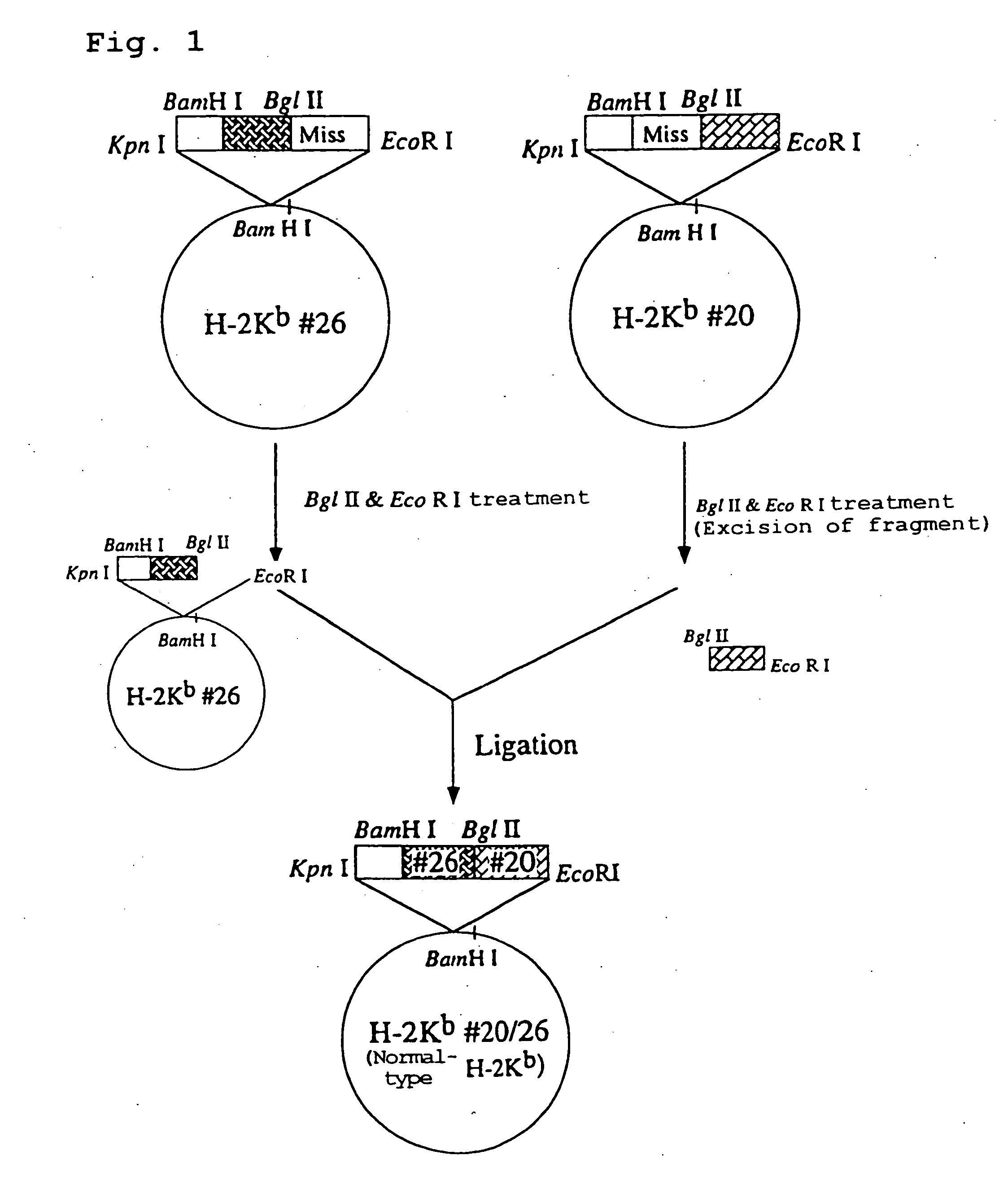
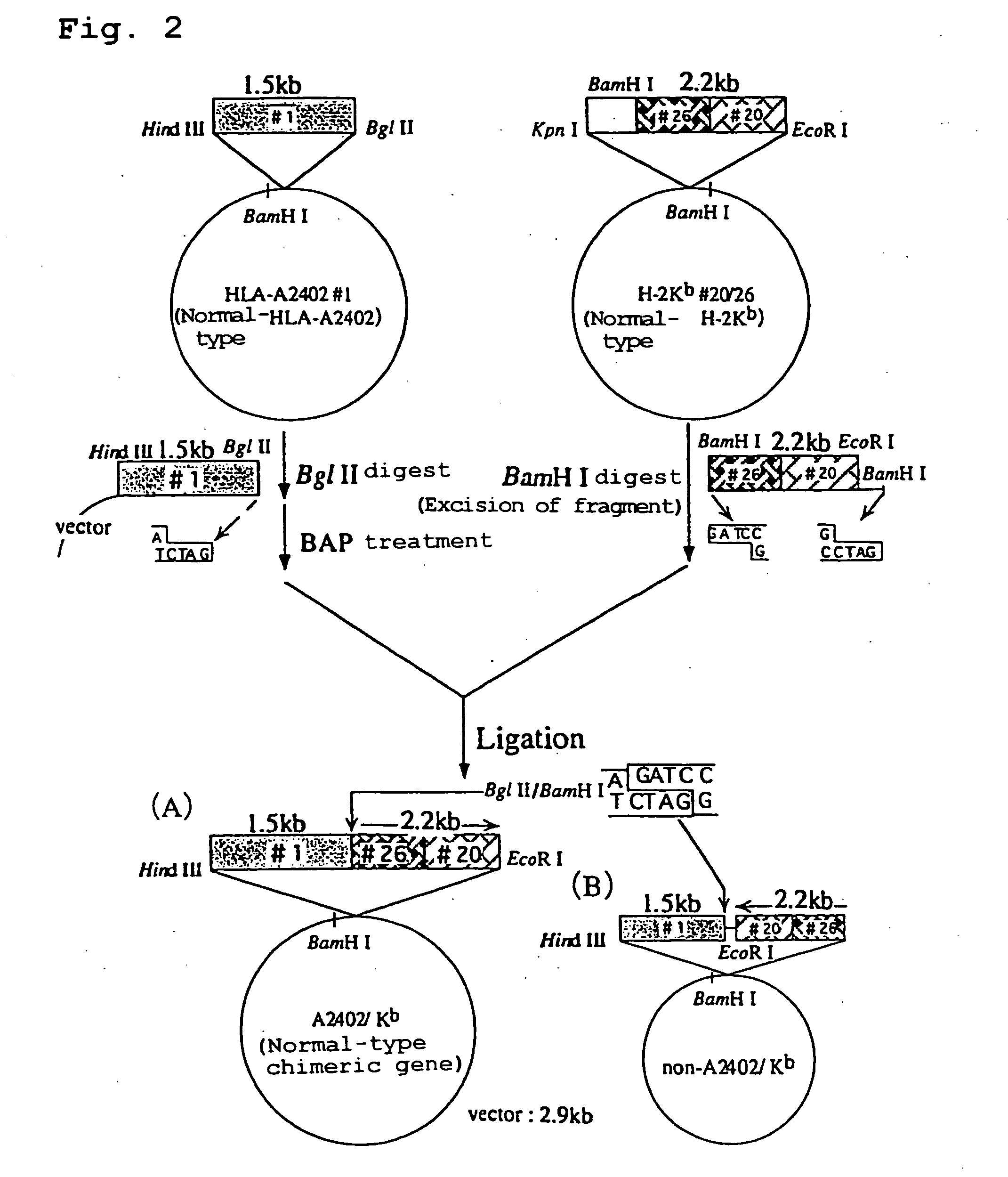
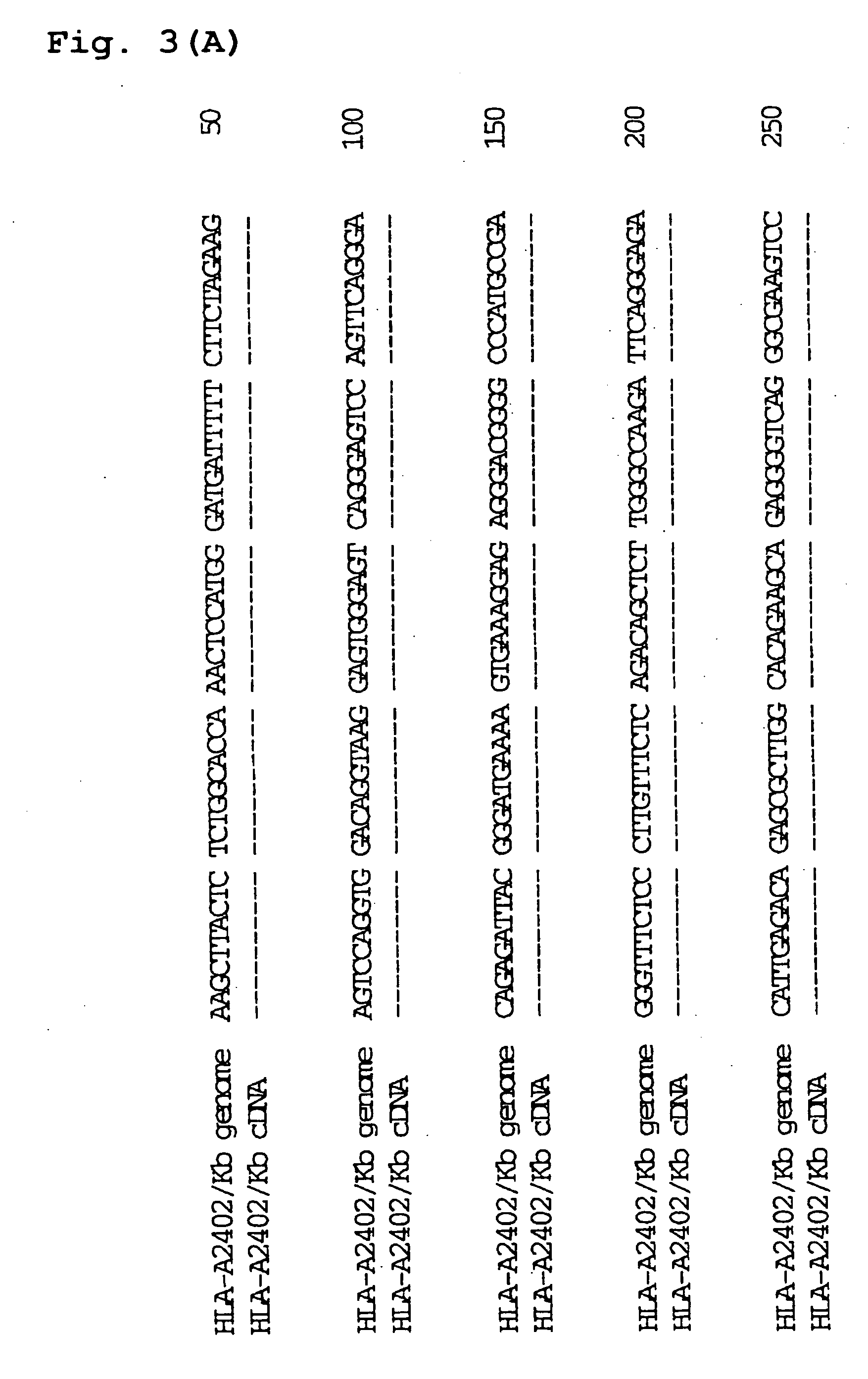
Transgenic animal expressing HLA-A24 and utilization thereof
The present invention relates to a non-human transgenic mammal which has had an HLA-A24 gene introduced and in which CTLs are induced when stimulated by an HLA-A24-binding antigen, a method of screening therapeutic or preventive agents for tumors or virus infections comprising administering a test substance to said transgenic non-human mammal and assaying and evaluating whether CTLs specific for the test substance are induced, an HLA-A24-binding tumor antigen peptide of PSA origin selected by said screening method, a chimera DNA (DNA construct) useful in the generation of said non-human transgenic mammal, and a host cell transformed by said chimera gene and use thereof.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
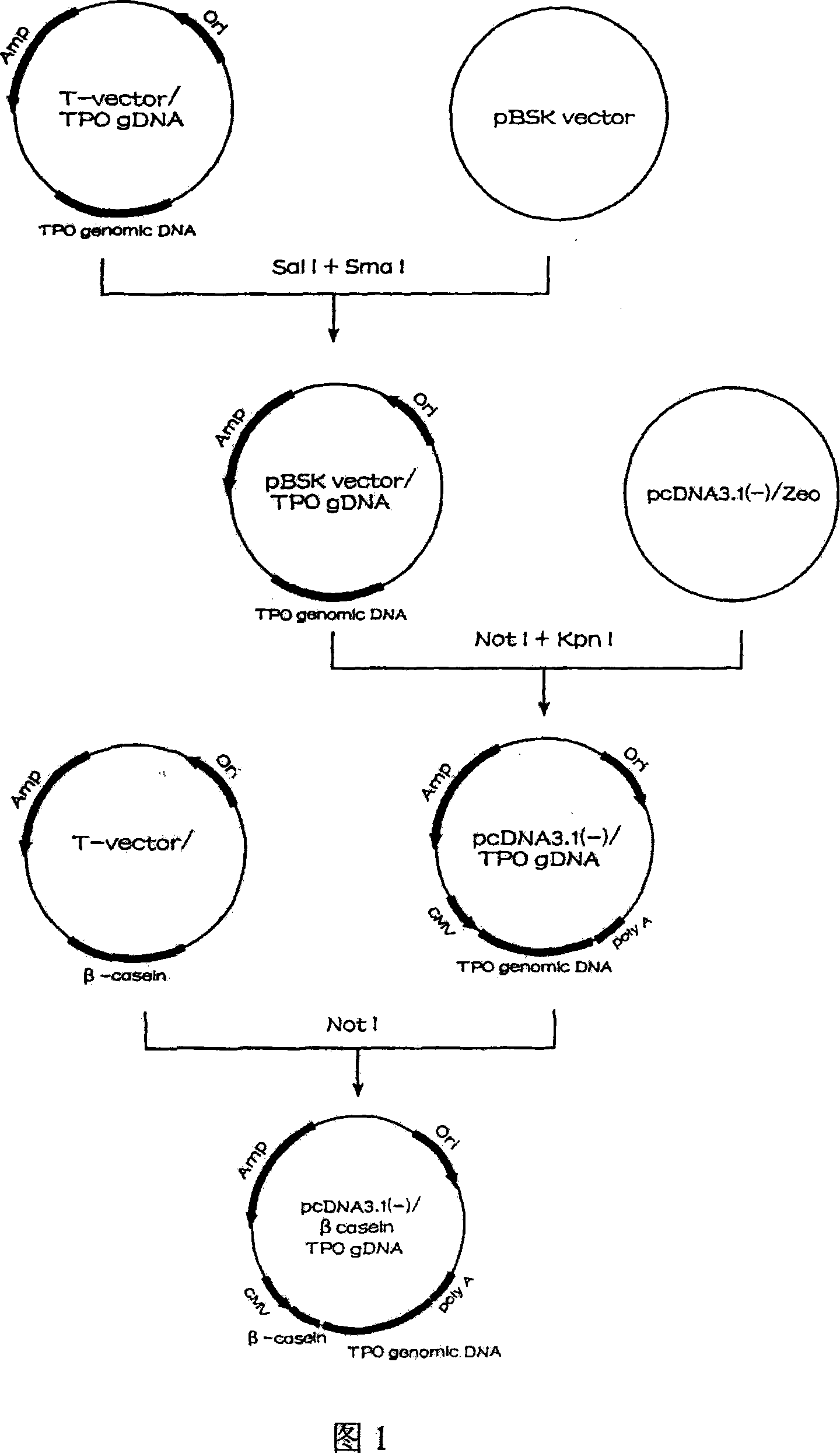
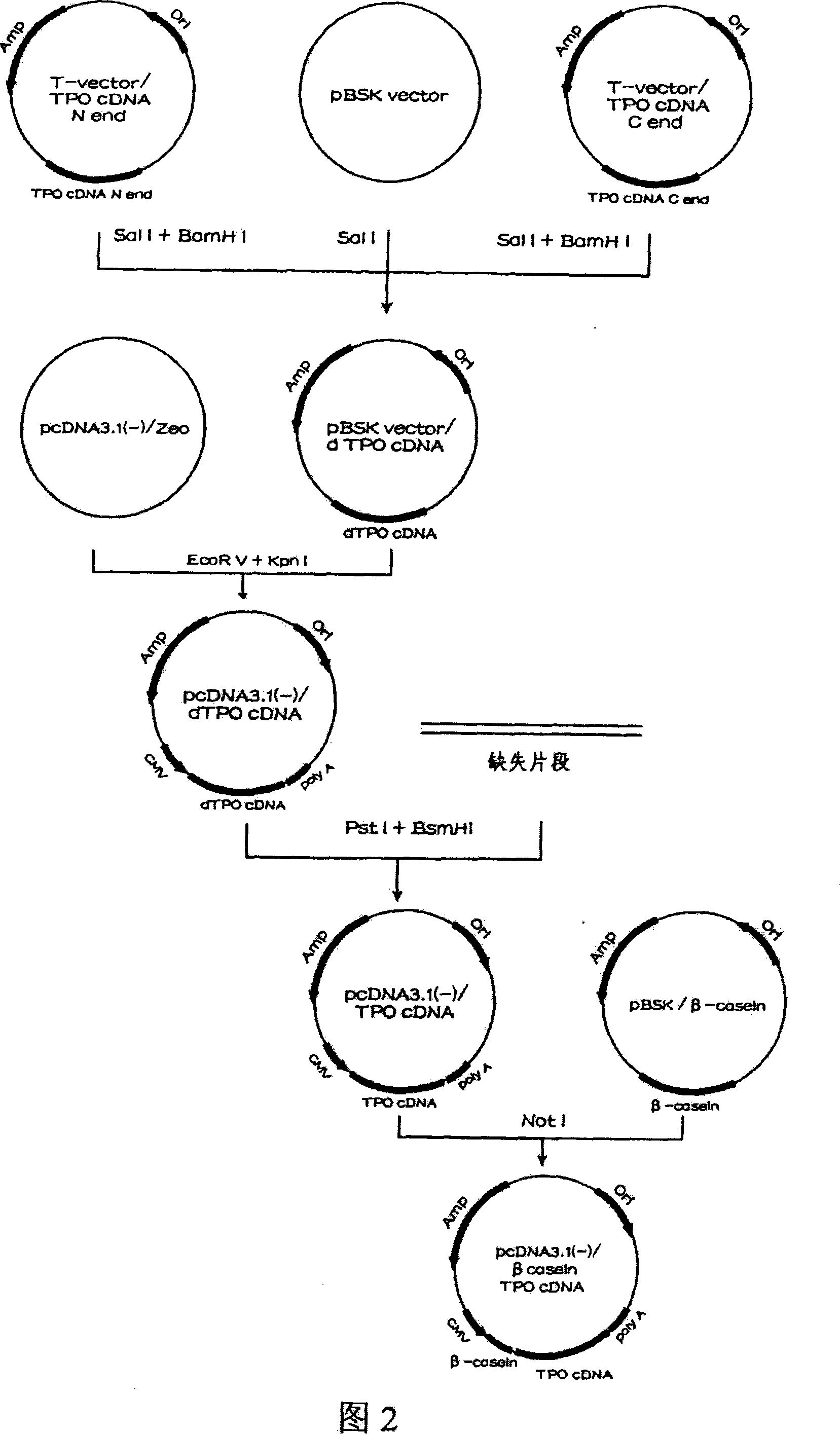
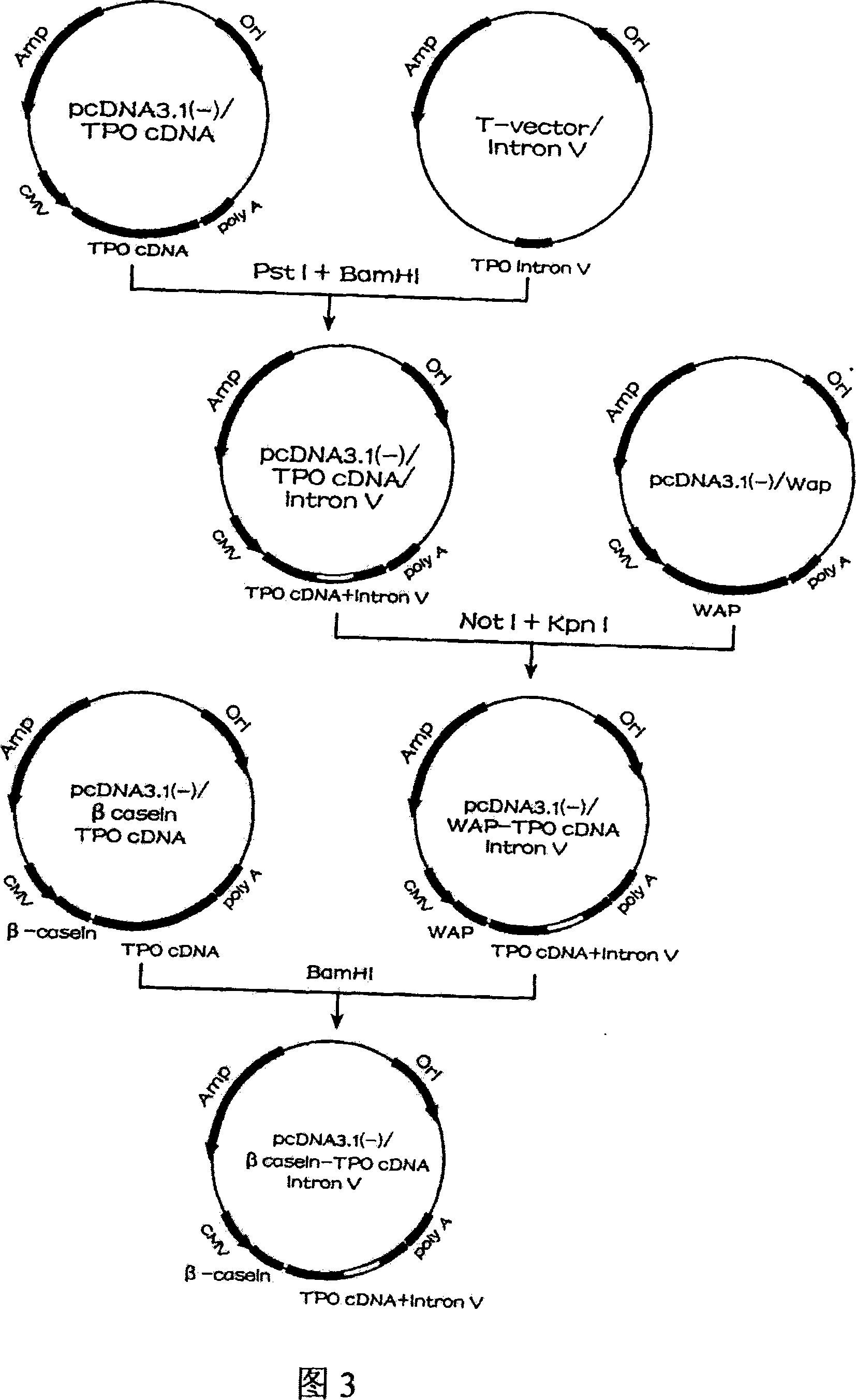
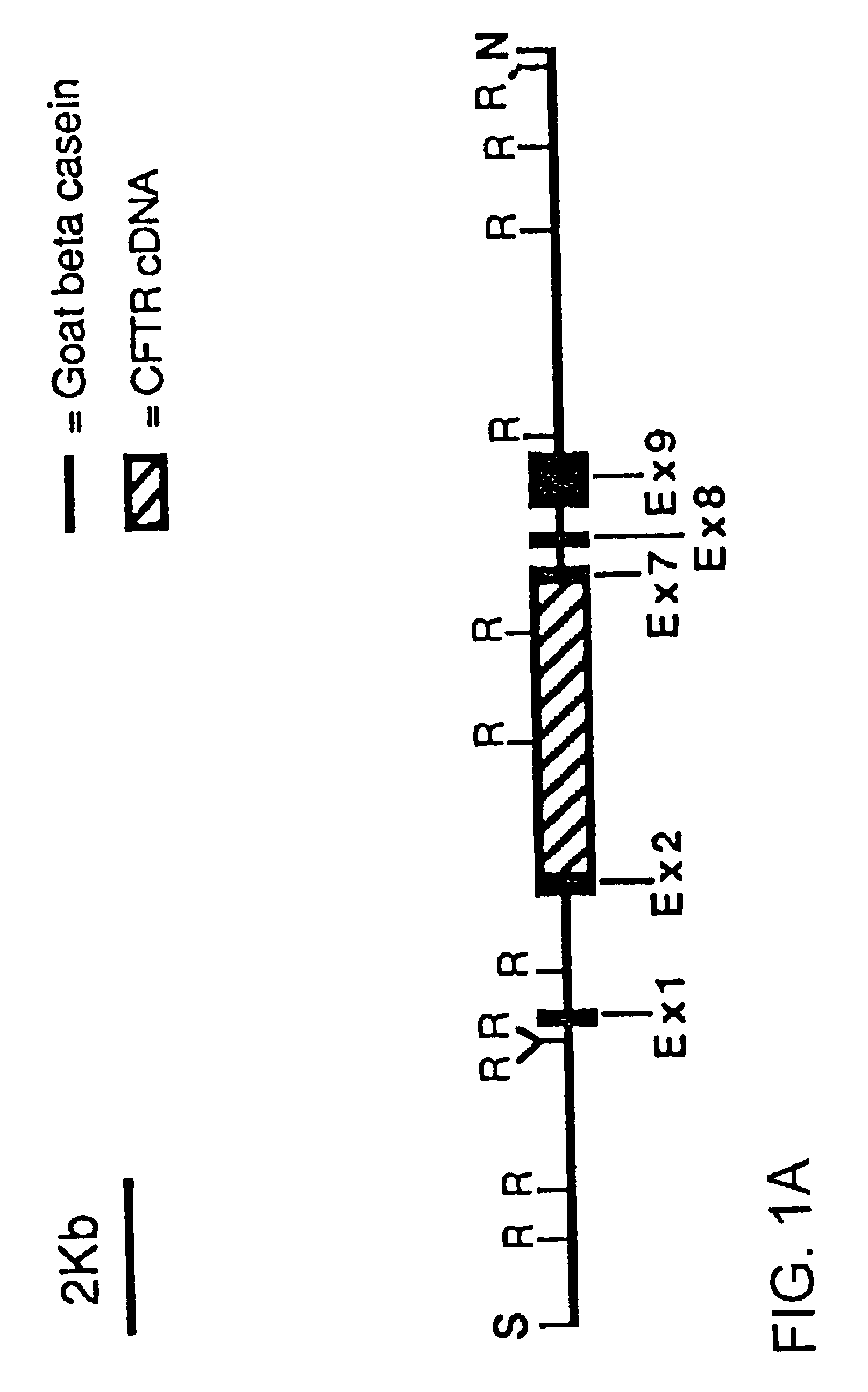
Human thrombopoietin expression vector and constructing method therefor
InactiveCN101250553AFermentationVector-based foreign material introductionHuman plateletThrombopoietin Gene
The invention belongs to the technical field of genetic engineering and transgenic engineering and in particular relates to a method for building mammary gland specific expression vectors of human thrombopoietin gene and goat beta-casein gene promoter. The vectors which are built are transferred to COS-7 and HC-11 cells and expressed on eukaryotic cell level verification hTPO. The built mammary gland specific expression vectors of the human thrombopoietin gene and the goat beta-casein gene promoter are applied to prepare a mammary gland bioreactor of transgenic animals and are expressed with high level in the milk of transgenic mammalian, and the vectors provide useful drug protein-hTPO for clinic. The mammary gland specific expression vectors of human thrombopoietin gene and goat beta-casein gene promoter which are provided by the invention provide a foundation for further research and development.
Owner:上海医学遗传研究所 +2
Transgenic mammals and methods of use thereof
ActiveUS20170306352A1Hybrid immunoglobulinsVector-based foreign material introductionTherapeutic antibodyMammal
Owner:TRIANNI INC
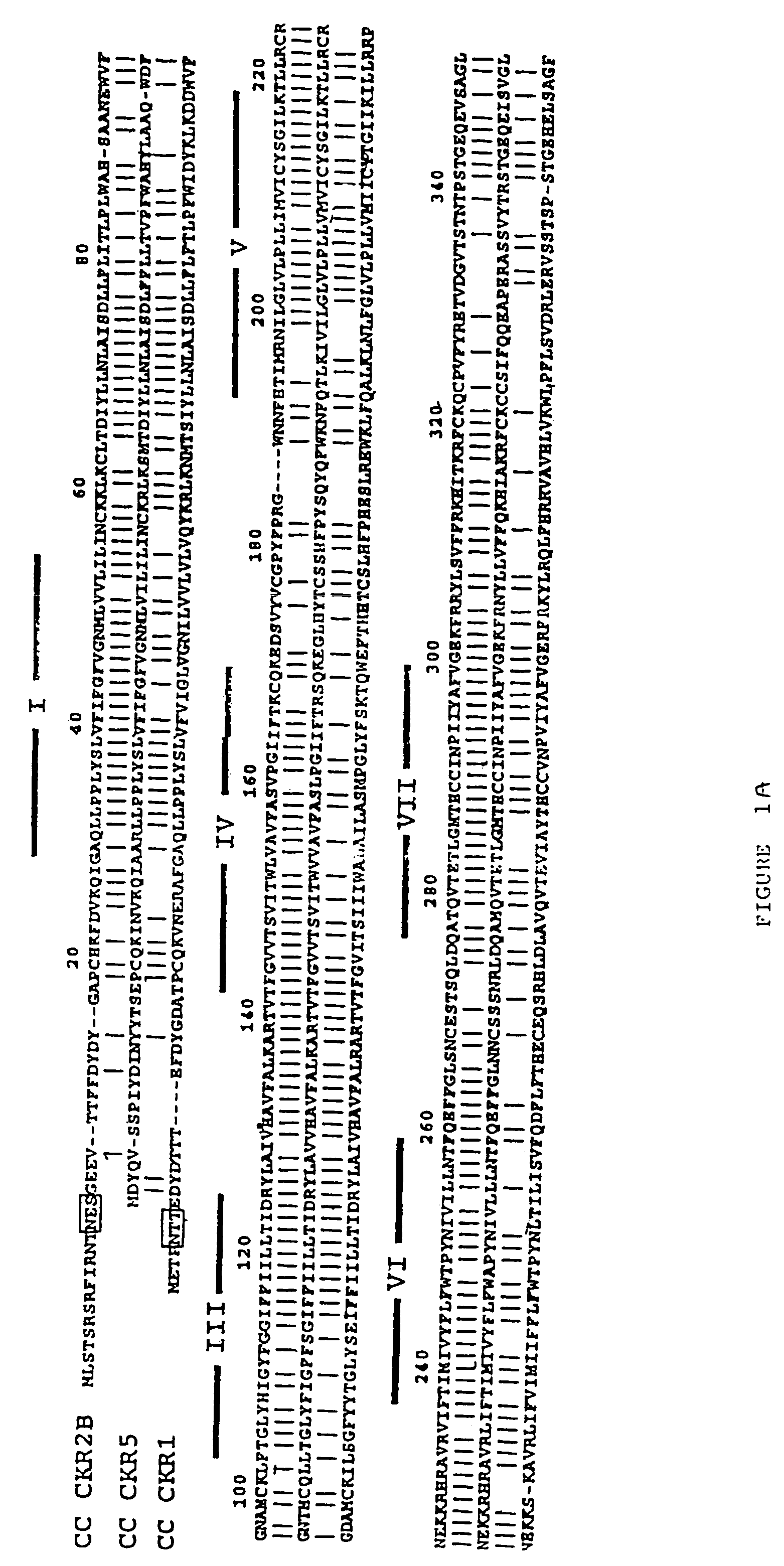
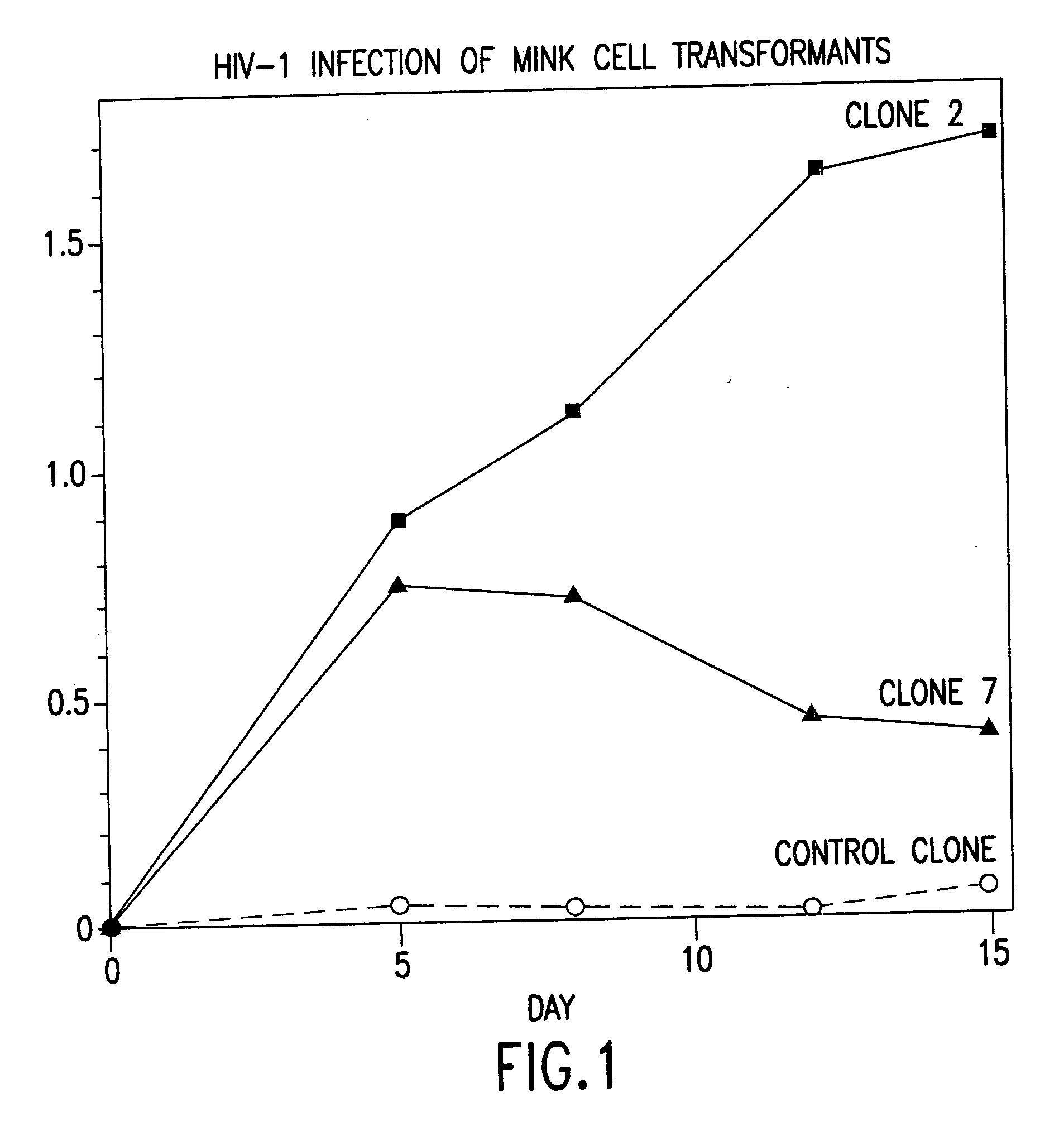
CC chemokine receptor 5 DNA, new animal models and therapeutic agents for HIV infection
InactiveUS7151087B2Effective in regulating monocyte accumulationEffective in regulating activationBiocidePeptide/protein ingredientsChemokine receptor D6Mammal
The susceptibility of human macrophages to human immunodeficiency virus (HIV) infection depends on cell surface expression of the human CD4 molecule and CC cytokine receptor 5. CCR5 is a member of the 7-transmembrane segment superfamily of G-protein-coupled cell surface molecules. CCR5 plays an essential role in the membrane fusion step of infection by some HIV isolates. The establishment of stable, nonhuman cell lines and transgenic mammals having cells that coexpress human CD4 and CCR5 provides valuable tools for the continuing research of HIV infection. In addition, antibodies which bind to CCR5, CCR5 variants, and CCR5-binding agents, capable of blocking membrane fusion between HIV and target cells represent potential anti-HIV therapeutics for macrophage-tropic strains of HIV.
Owner:UNITED STATES OF AMERICA
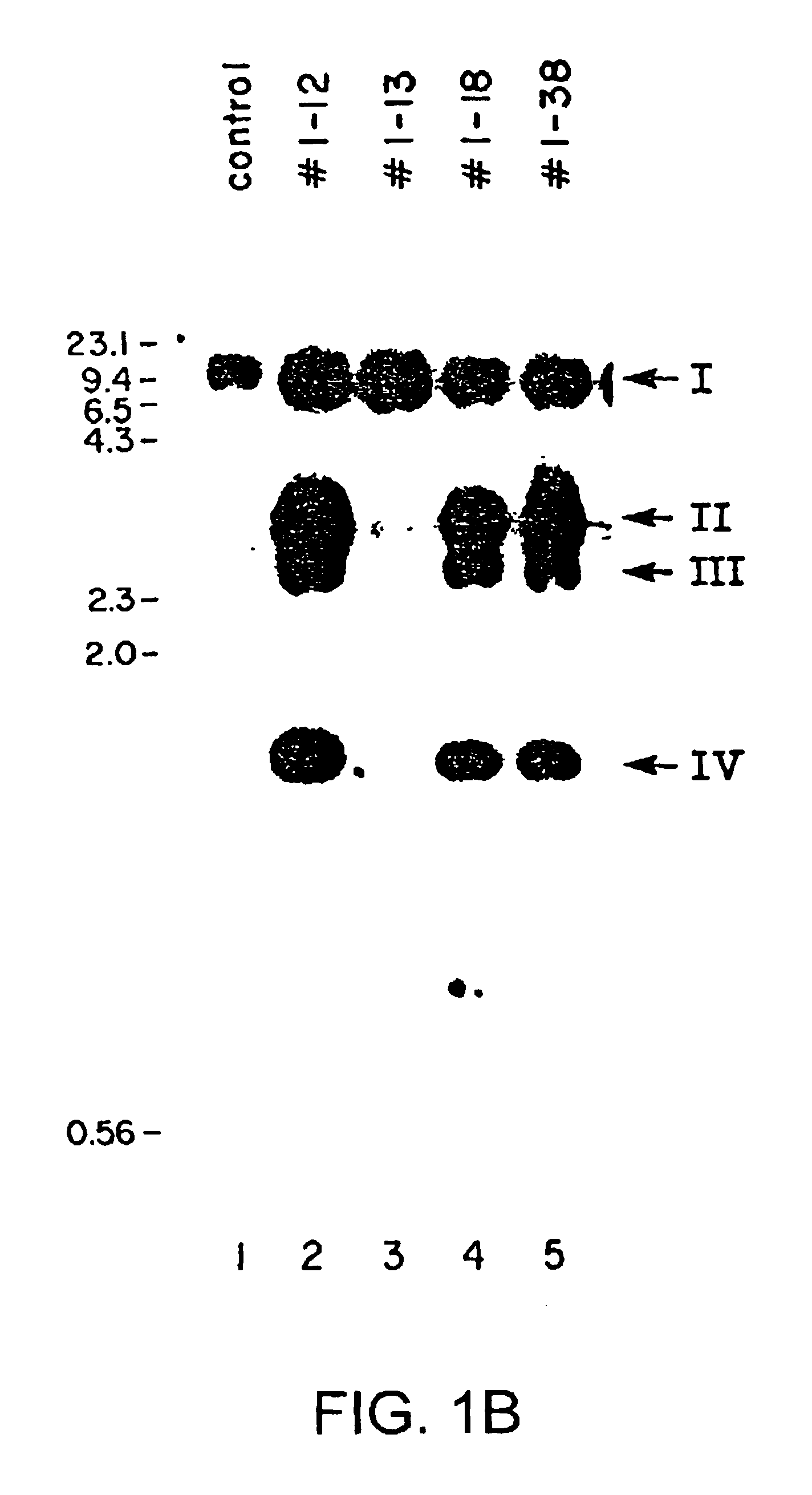
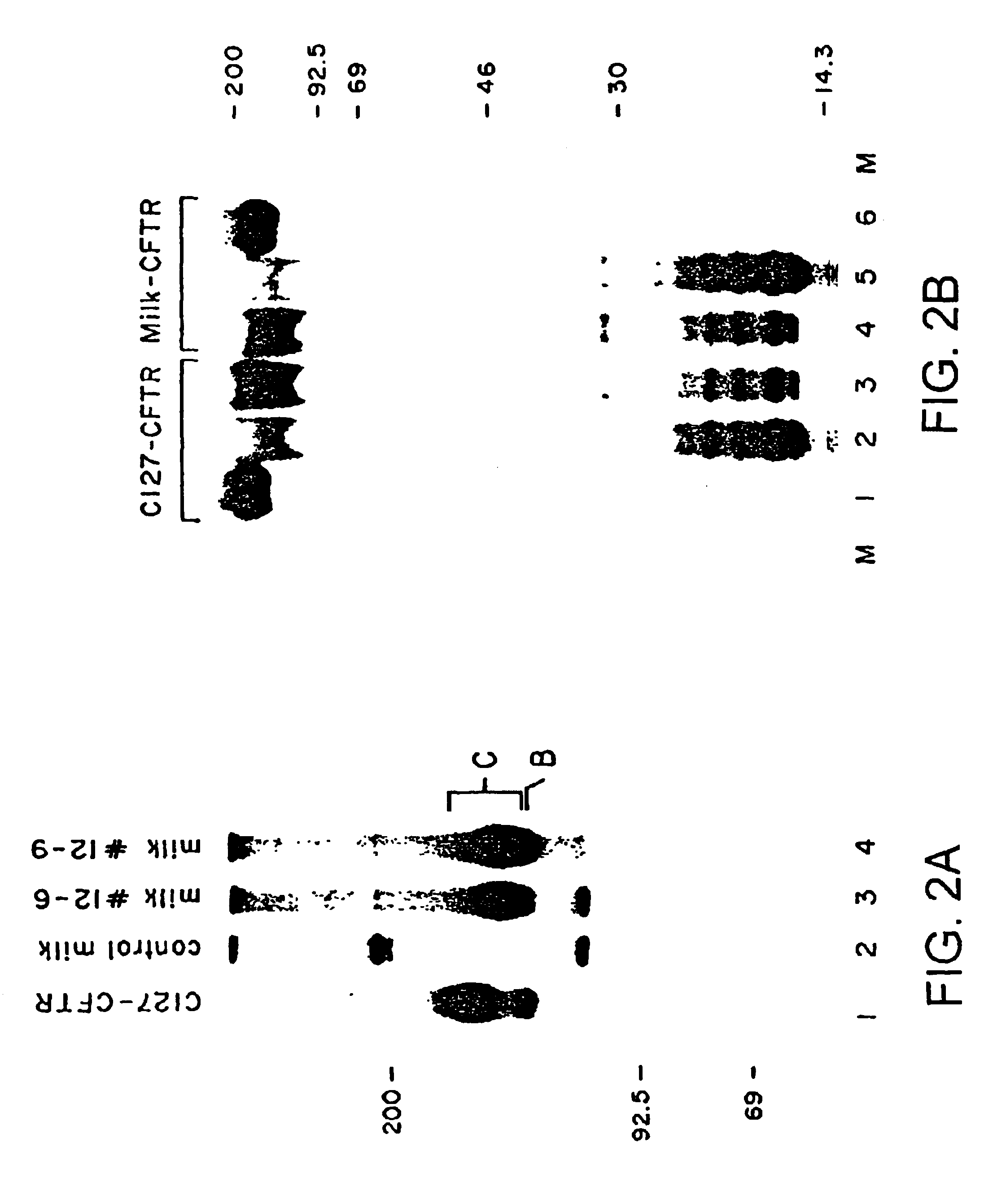
Production of membrane proteins in the milk of transgenic mammals
InactiveUS6743966B2Cell receptors/surface-antigens/surface-determinantsFermentationBiotechnologyMammal
Production of proteins not normally secreted through conventional pathways such as membrane proteins including, for example, CFTR associated with cystic fibrosis, is now made possible by collection of such protein from the milk of lactating transgenic animals.
Owner:GENZYME CORP
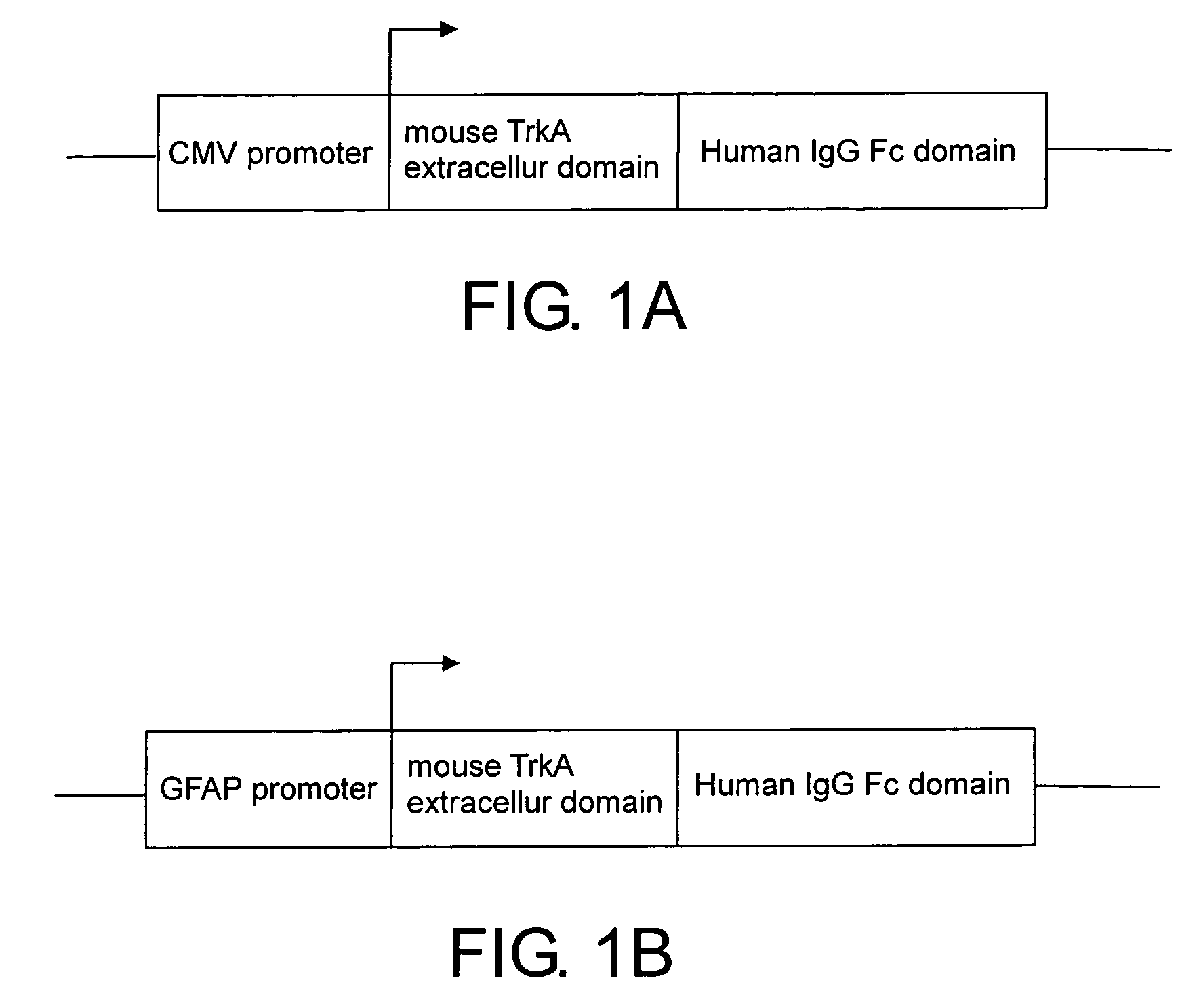
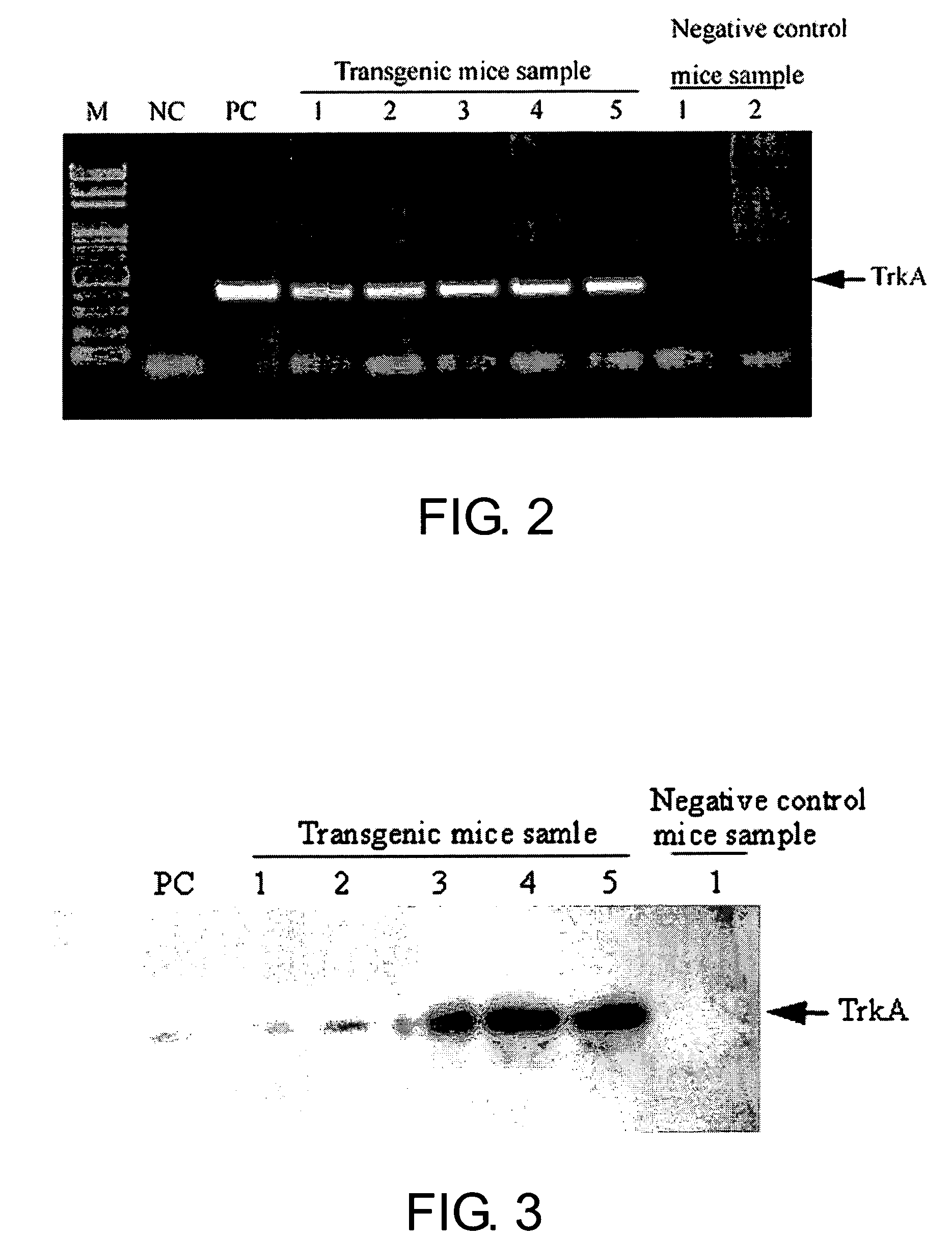
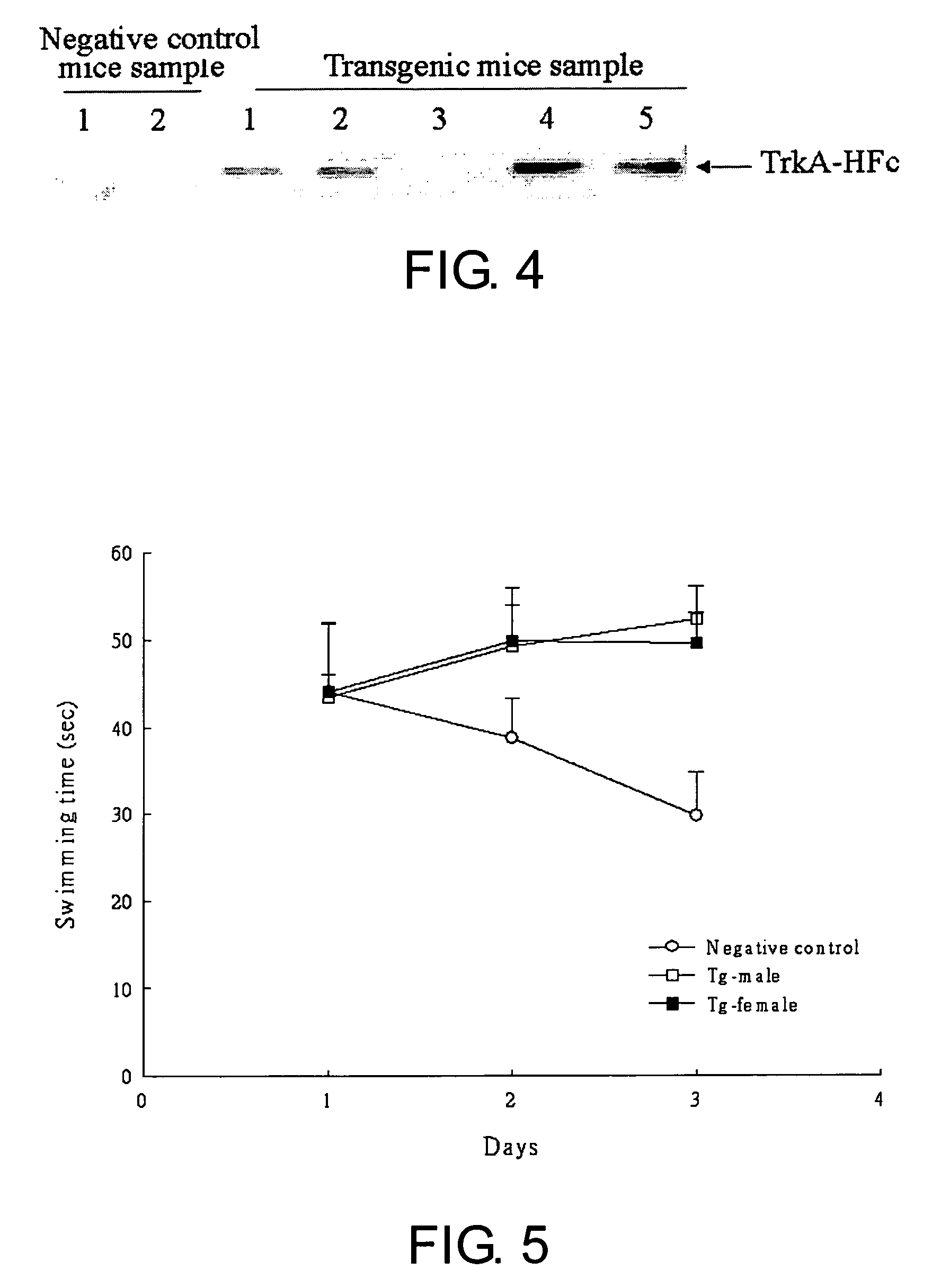
Neurodegenerative non-human transgenic mammal expressing TrkA fusion protein
The present invention provides a neurodegenerative, non-human transgenic mammal whose cells contain a TrkA-hFc transgene for encoding at least a TrkA fusion protein and the use thereof.
Owner:IND TECH RES INST
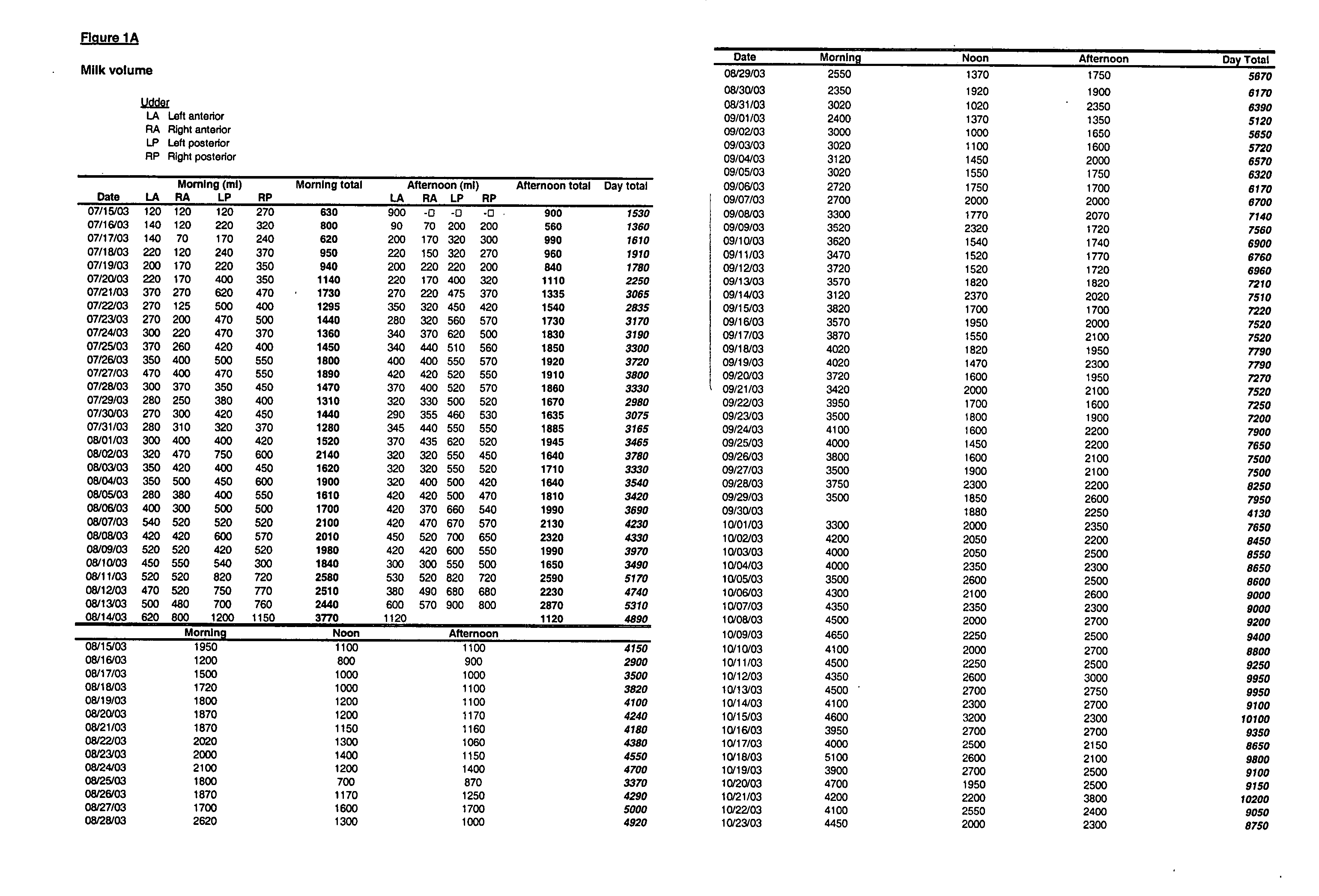
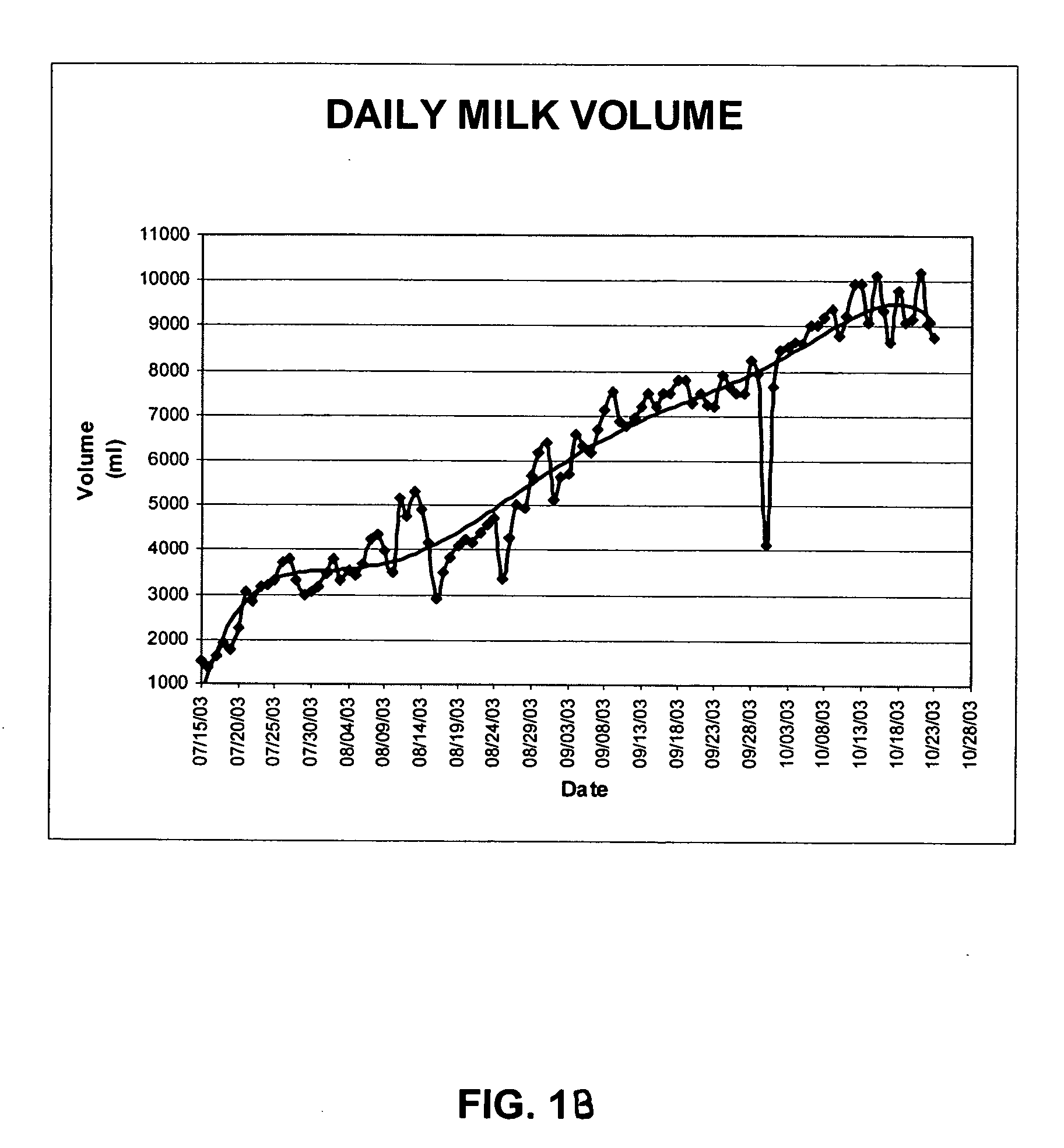
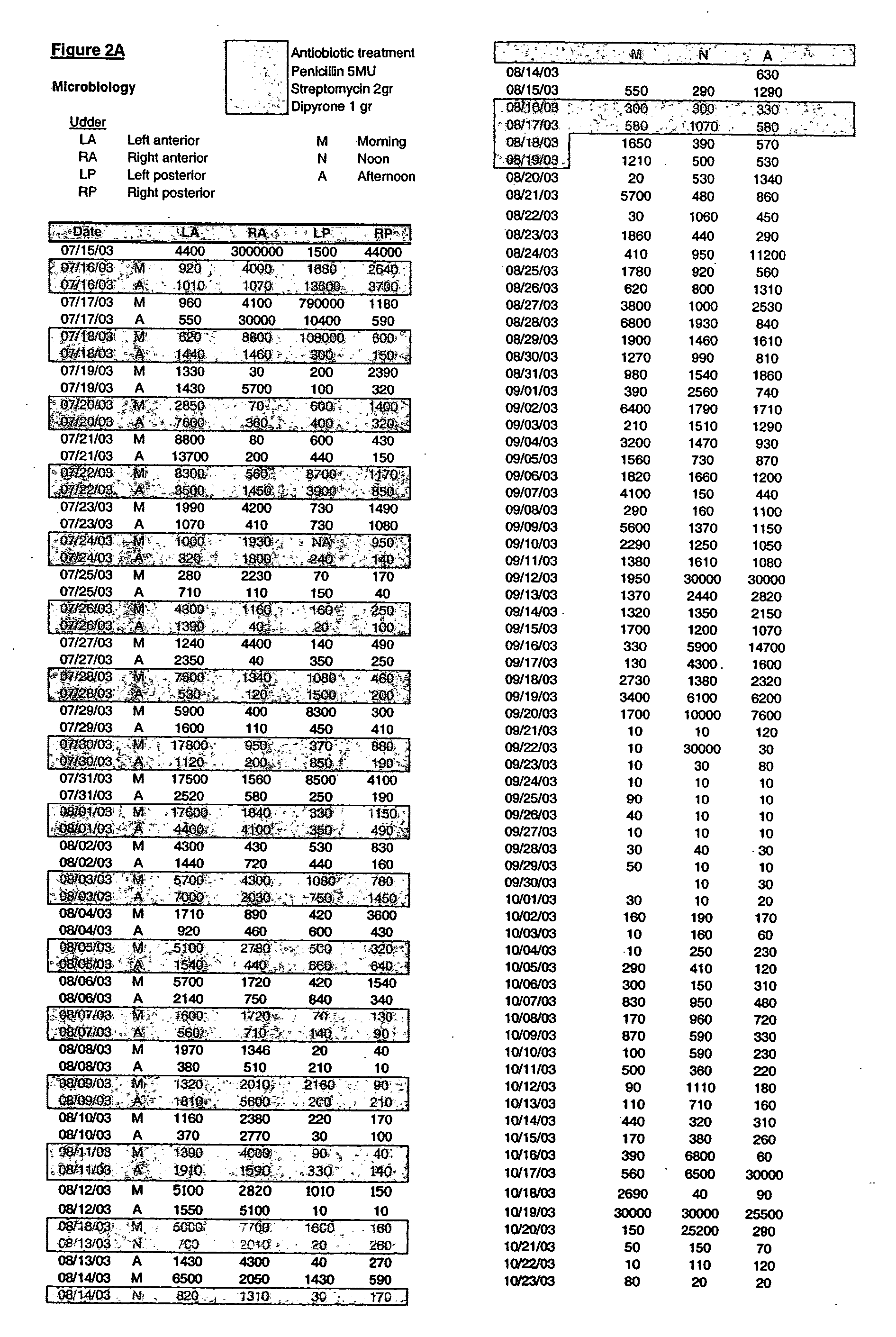
Process of making transgenic mammals that produce exogenous proteins in milk and transgenic mammals produced thereby
InactiveUS20050177878A1Improve the level ofVectorsPeptide preparation methodsBovine oocyteSomatotropic hormone
The invention relates to a method of producing a protein of interest, comprising making a non-human transgenic mammal that produces said protein in its milk, obtaining said milk from the non-human transgenic mammal and purifying said protein of interest from the milk. Transgenic bovine animals were generated, which are able to produce human growth hormone in mammary glands. The method involves cloning of a genetic construct encoding hGH gene and beta casein promoter conveniently in an expression vector. It also includes transfection procedures into fetal bovine somatic cells, generally fibroblasts, and the nuclear transfer into enucleated bovine oocytes, generating thus transgenic embryos. The method also includes other procedures to generate transgenic embryos for the further expansion of the transgenic herd, such as the subcloning of transgenic female bovines, the superovulation of transgenic cows and their insemination with semen from a non-transgenic or a transgenic male bovine, and the superovulation of non-transgenic cows and their insemination with semen from a transgenic male bovine. Afterwards, transgenic embryos give rise to transgenic cattle that produce human growth hormone in huge amounts in their milk, from which the hormone is completely purified and analysed to fulfill all the requirements for the manufacture of a pure biopharmaceutical product.
Owner:STERRENBELD BIOTECH NORTH AMERICA
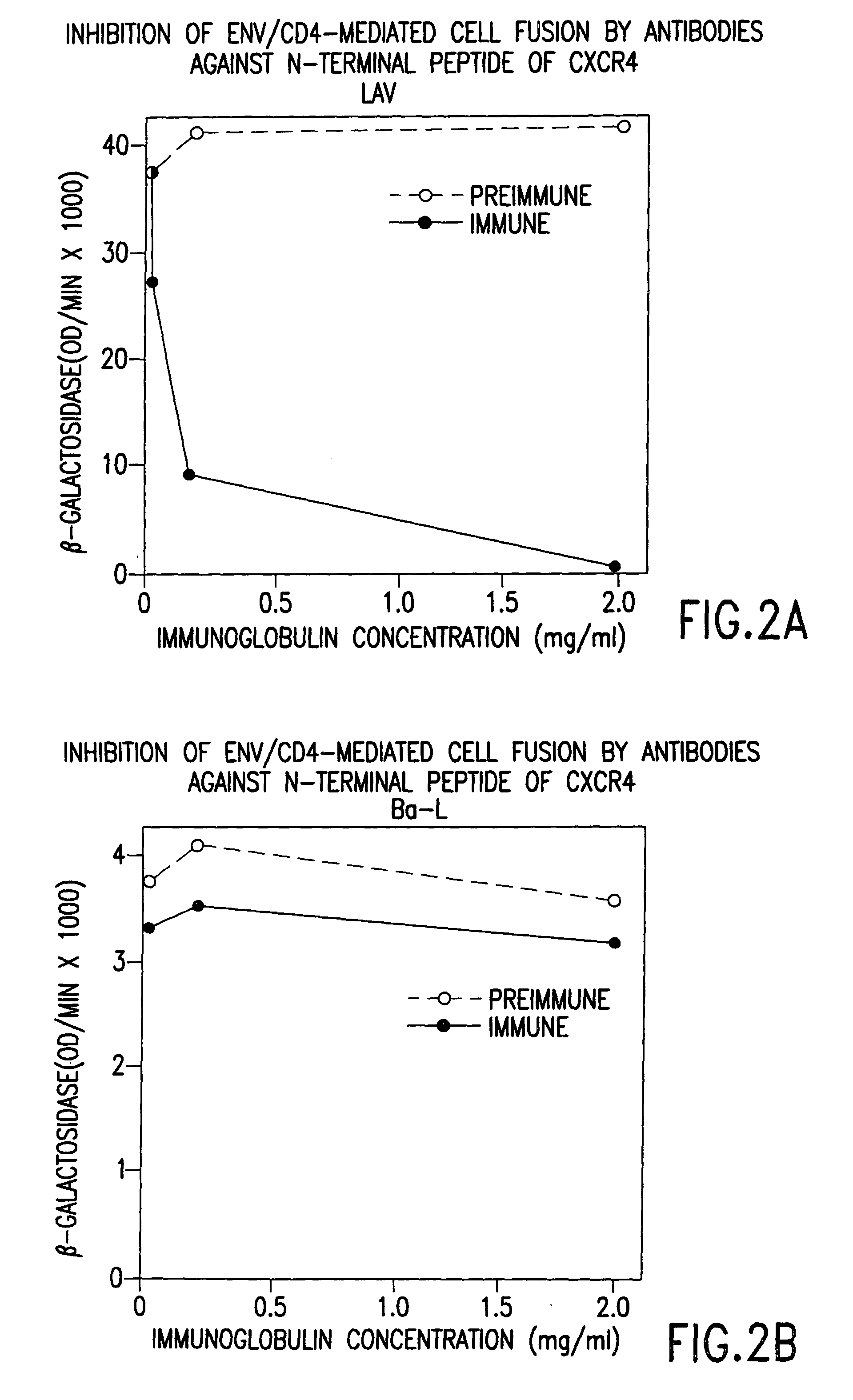
Cells expressing both human CD4 and a human fusion accessory factor associated with HIV infection
The susceptibility to human immunodeficiency virus (HIV) infection depends on the cell surface expression of the human CD4 molecule and a human fusion accessory factor associated with HIV infection (CXCR4). CXCR4 is a member of the 7-transmembrane segment superfamily of G-protein-coupled cell surface molecules. CXCR4 plays an essential role in the membrane fusion step of HIV infection. The establishment of stable, nonhuman cell lines and transgenic mammals having cells that coexpress human CD4 and CXCR4 provides valuable tools for the continuing research of HIV infection and the development of more effective anti-HIV therapeutics. In addition, antibodies against CXCR4, isolated and purified peptide fragments of CXCR4, and CXCR4-binding biologic agents, capable of blocking membrane fusion between HIV and target cells represent potential anti-HIV therapeutics.
Owner:BERGER EDWARD +3
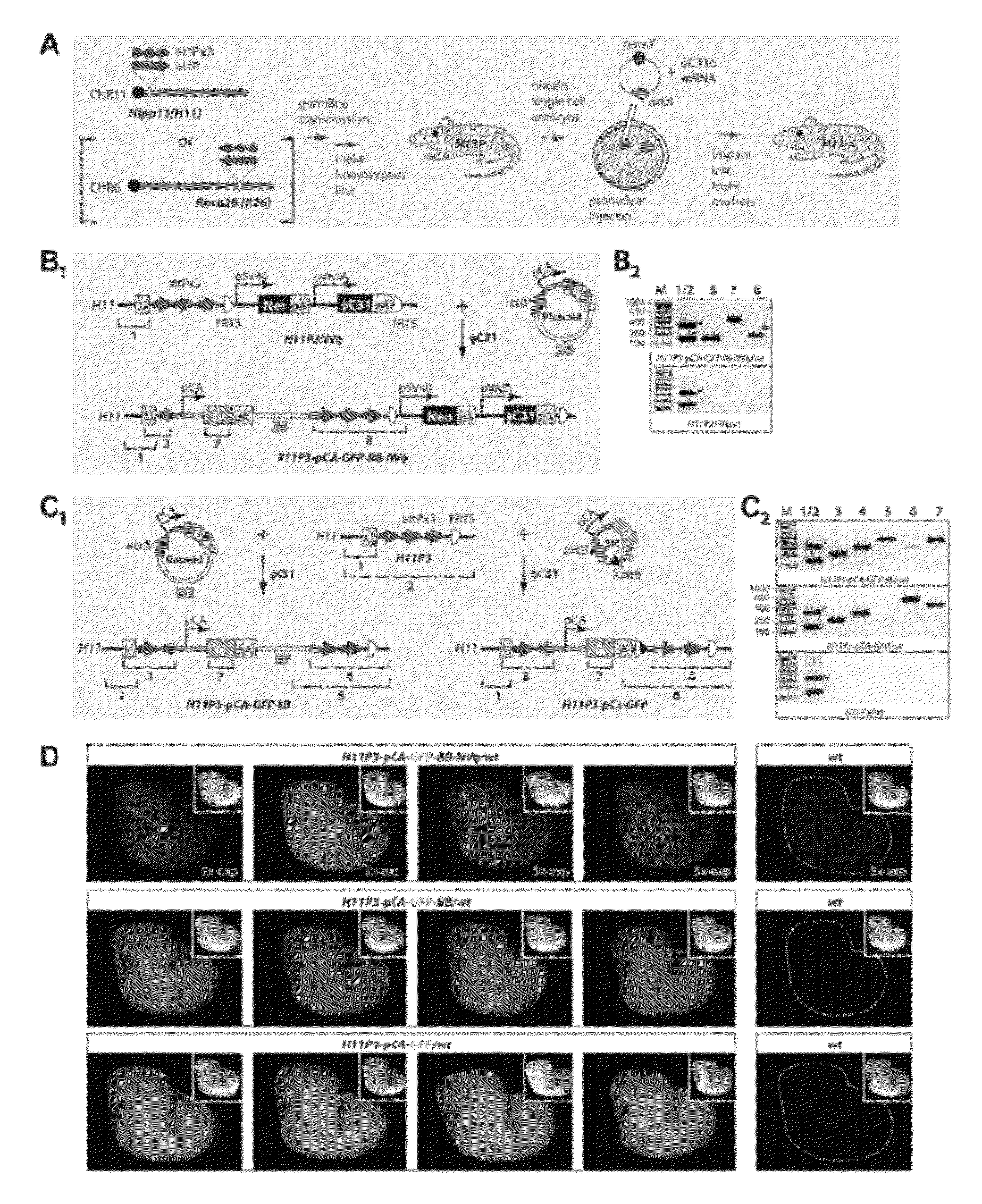
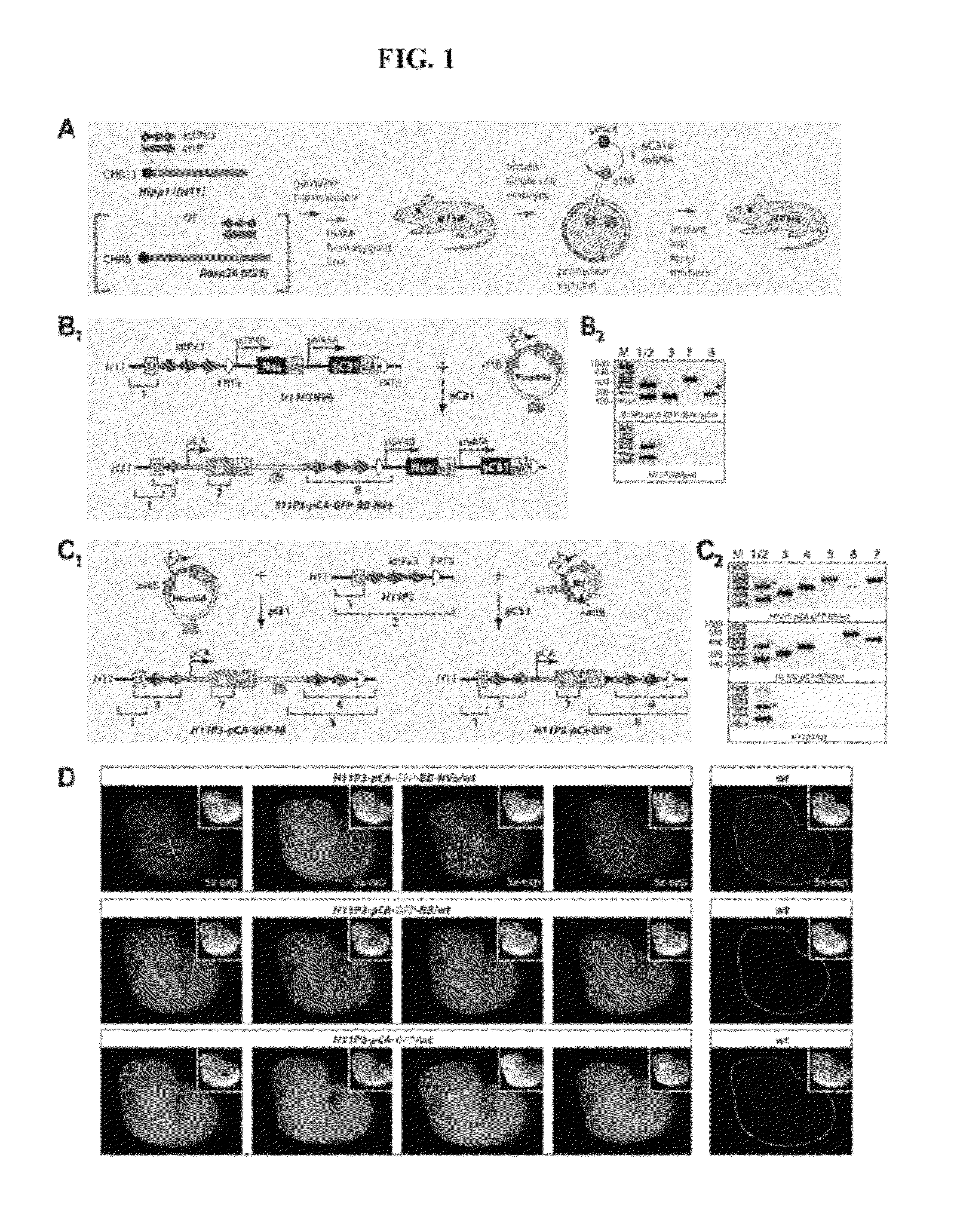
Site-Directed Integration of Transgenes in Mammals
ActiveUS20120124686A1Easy to understandNucleic acid vectorOther foreign material introduction processesMammalian genomeSite-specific recombination
The present disclosure provides a method of making a mammal (e.g., a rodent, such as a mouse) by integrating an intact polynucleotide sequence into a specific genomic locus of the mammal to result in a transgenic mammal. A transgenic mammal made by the methods of the present disclosure would contain a known copy number (e.g., one) of the inserted polynucleotide sequence at a predetermined location. The method involves introducing a site-specific recombinase and a targeting construct, containing a first recombination site and the polynucleotide sequence of interest, into the mammalian cell. The genome of the cell contains a second recombination site and recombination between the first and second recombination sites is facilitated by the site-specific, uni-directional recombinase. The result of the recombination is site-specific integration of the polynucleotide sequence of interest in the genome of the mammal. This inserted sequence is then also transmitted to the progeny of the mammal.
Owner:UNIVERSITY OF OREGON +1
Method for the production of fusion proteins in transgenic mammal milk
InactiveUS7550263B2Efficient productionLow costPeptide/protein ingredientsAntipyreticPost translationalPurification methods
Desirable fusion proteins can be produced in and purified from the milk of transgenic animals. The peptides are made as fusion proteins with a suitable fusion partner such as human alpha-fetoprotein. The fusion partner protein acts to promote and increase the half-life of the overall molecule as well as having therapeutic effects on its own. The fusion protein is typically produced through the use of transgenic animals and can be purified away from the now the milk or other bodily fluid of such an animal by an affinity purification method. A particular advantage of producing peptides via this route, in addition to the obvious advantages of high yield and biocompatibility, is that specific post-translational modifications, such as carboxy terminal amidation, can be performed in the mammary gland. Biologically active polypeptides comprising a therapeutically active polypeptide fused to human alpha-fetoprotein fragment or a variant thereof, methods for the preparation thereof, nucleotide sequences encoding such fusion polypeptides, expression cassettes comprising such nucleotide sequences, self-replicating plasmids containing such expression cassettes, and pharmaceutical compositions containing said fusion polypeptides.
Owner:GTC BIOTHERAPEUTICS INC
Method of purifying recombinant MSP 1-42 derived from Plasmodium falciparum
InactiveUS20060130159A1Minimize concentration polarizationReduce fluxDepsipeptidesPeptide preparation methodsBiotechnologySource material
Owner:GTC BIOTHERAPEUTICS INC
Cloning using donor nuclei from differentiated fetal and adult cells
InactiveUS20020010949A1Rejection is prevented and reducedEliminate, orBiocideNervous disorderPresent methodNuclear transfer
An improved method of nuclear transfer involving the transplantation of donor differentiated cell nuclei into enucleated oocytes of the same species as the donor cell is provided. The resultant nuclear transfer units are useful for multiplication of genotypes and transgenic genotypes by the production of fetuses and offspring, and for production of isogenic CICM cells, including human isogenic embryonic or stem cells. Production of genetically engineered or transgenic mammalian embryos, fetuses and offspring is facilitated by the present method since the differentiated cell source of the donor nuclei can be genetically modified and clonally propagated.
Owner:UNIVERSITY OF MASSACHUSETTS AMHERST
Enhanced production of immunoglobulins
ActiveUS20170226162A1Improve efficiencyDetermination of immunoglobulin specificityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyMammalPlasma cell
Owner:TRIANNI INC
Enhanced production of immunoglobulins
Owner:TRIANNI INC
Universal stem cells
Owner:LAWMAN MICHAEL J P +1
Transgenic Reporter Mouse and Method for Use
InactiveUS20100122355A1High through-put screeningCompounds screening/testingVectorsMammalFluorescence
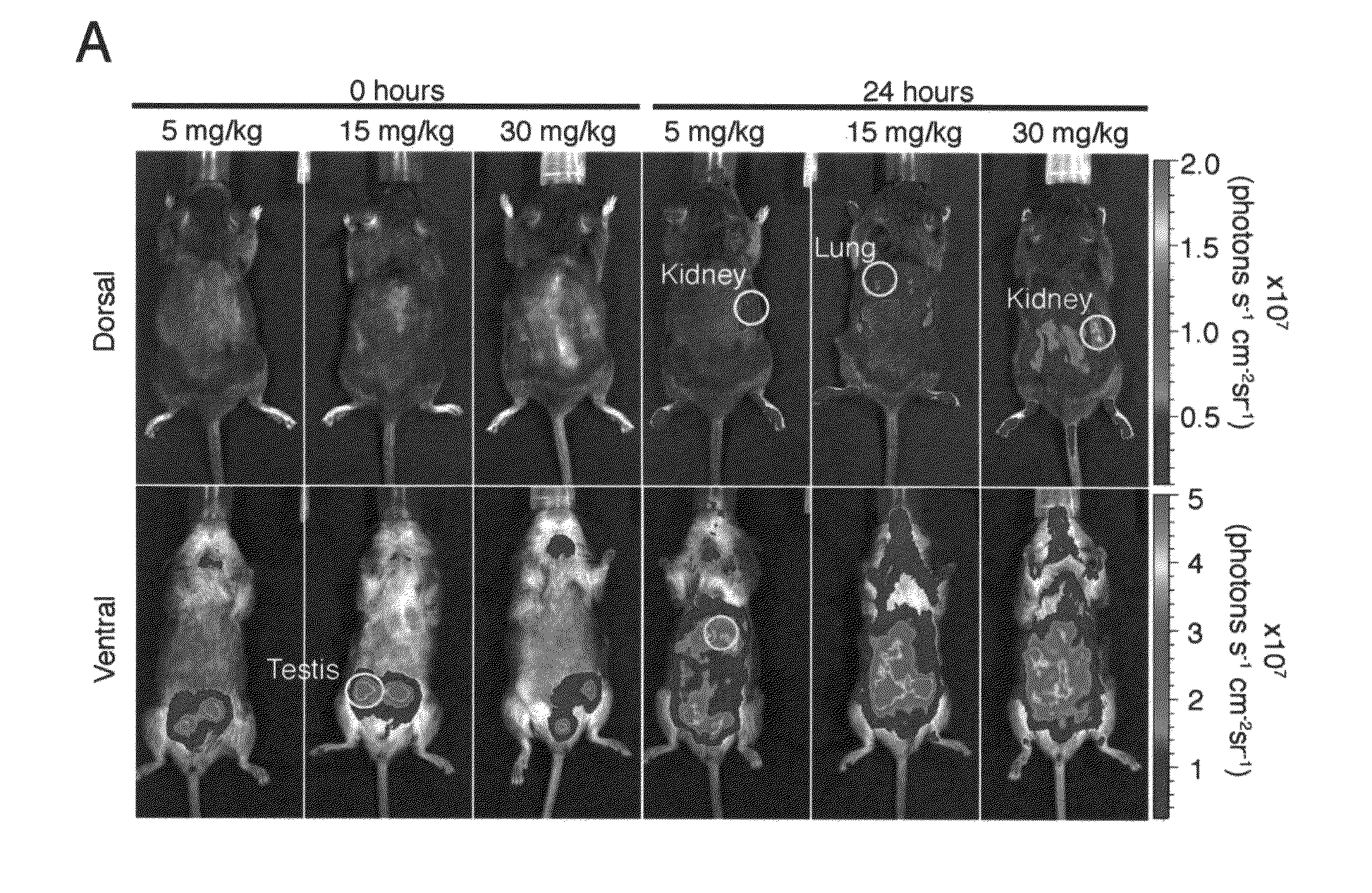
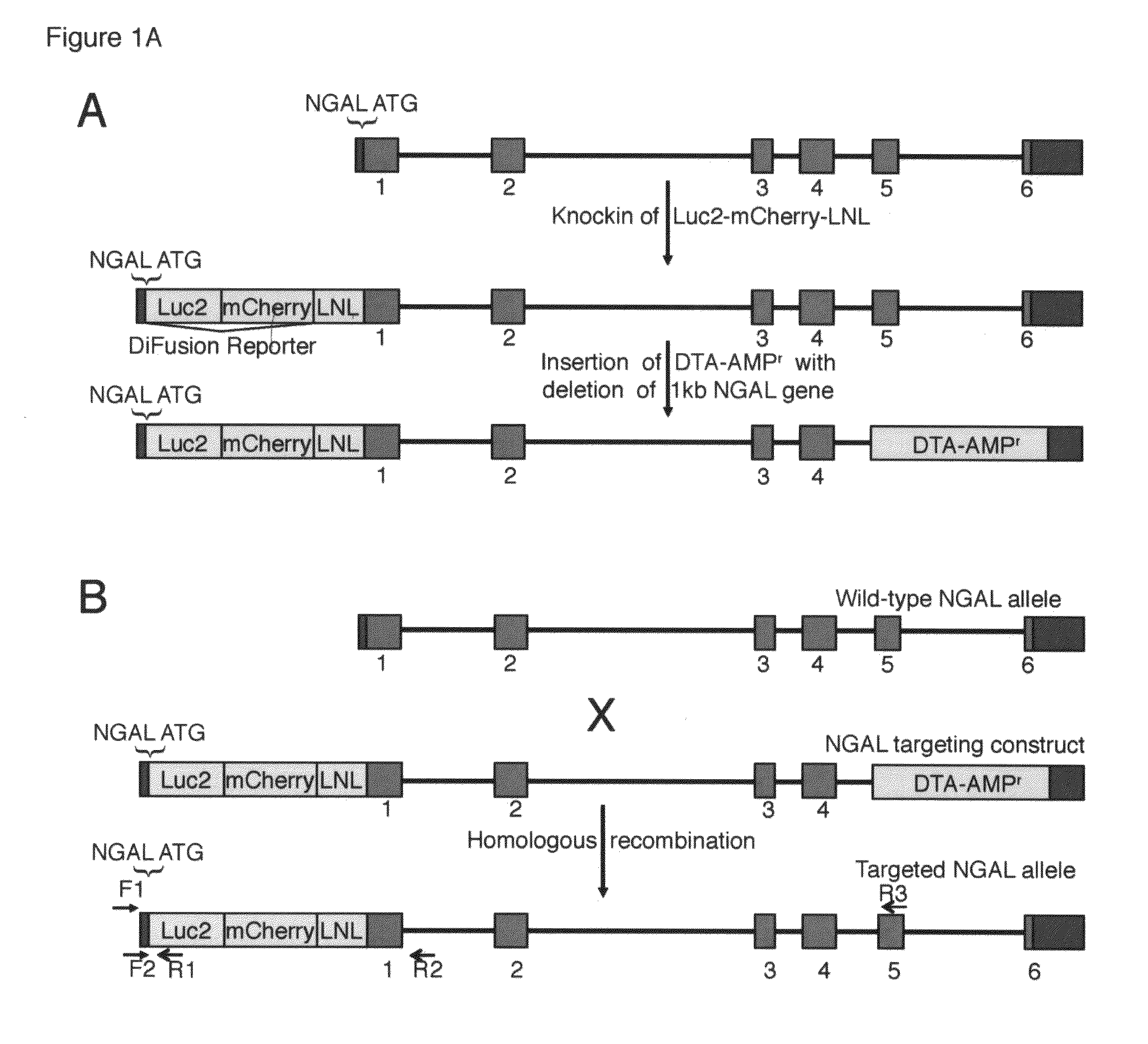
A transgenic mammal, including a transgenic mouse, whose genome comprises a transgene, said transgene comprises a neutrophil gelatinase-associated lipocalin (NGAL) promoter gene operably linked to at least one sequence encoding at least one of a fluorescent or bioluminescent protein, wherein the NGAL promoter gene expression in the mouse can be assayed by bioluminescence or fluorescence imaging.
Owner:PARAGAS NEAL +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com