C1 inhibitor produced in the milk of transgenic mammals
a technology of c1 inhibitor and transgenic mammals, which is applied in the field of recombinant genetics and medicine, can solve the problems of ineffectiveness, life-threatening situation of laryngeal edema, and painful swelling of intestinal mucous, and achieve the effect of promoting the expression of dna segments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Transgenes
a. Overlapping Genomic Constructs (CINH1)
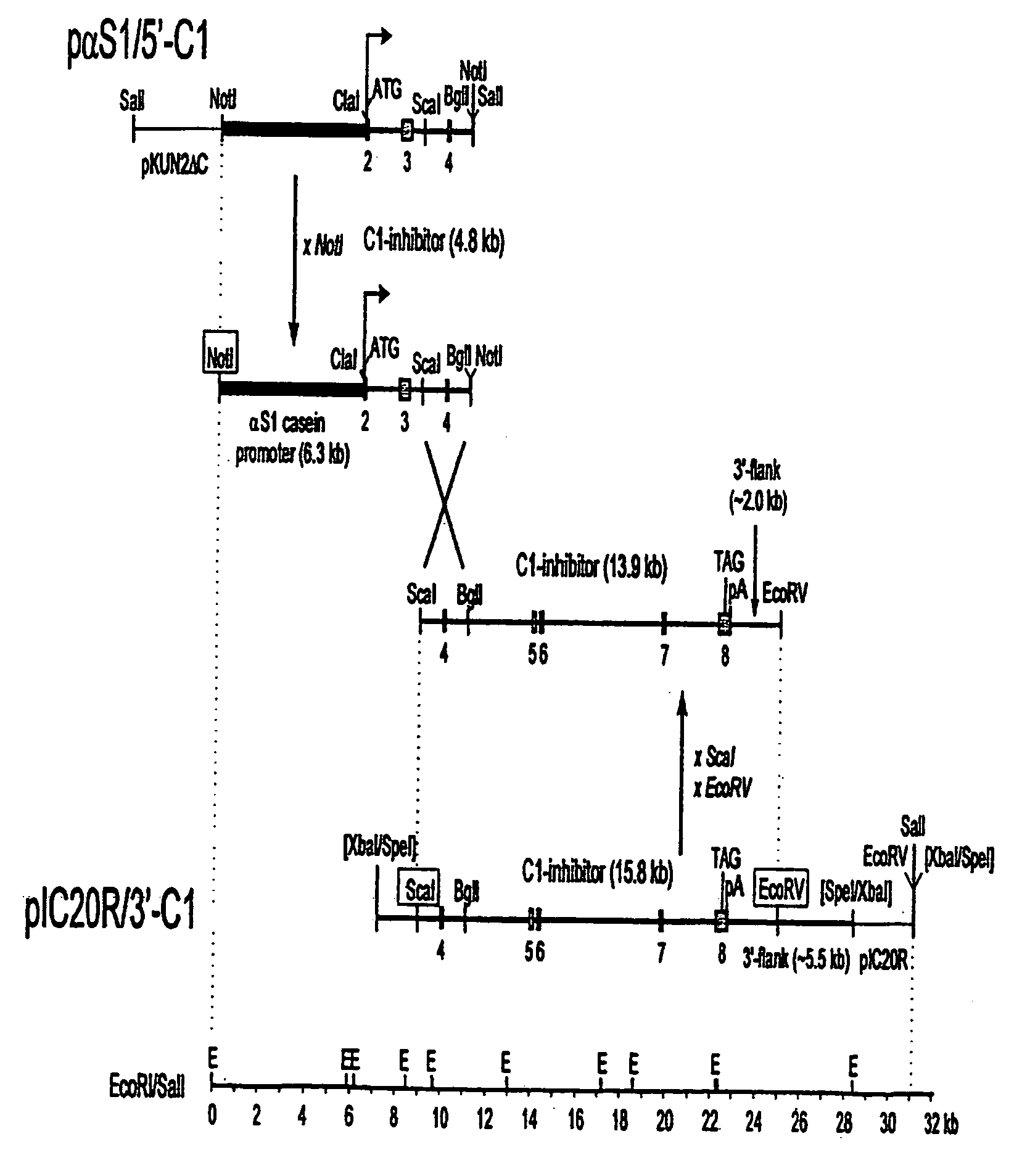
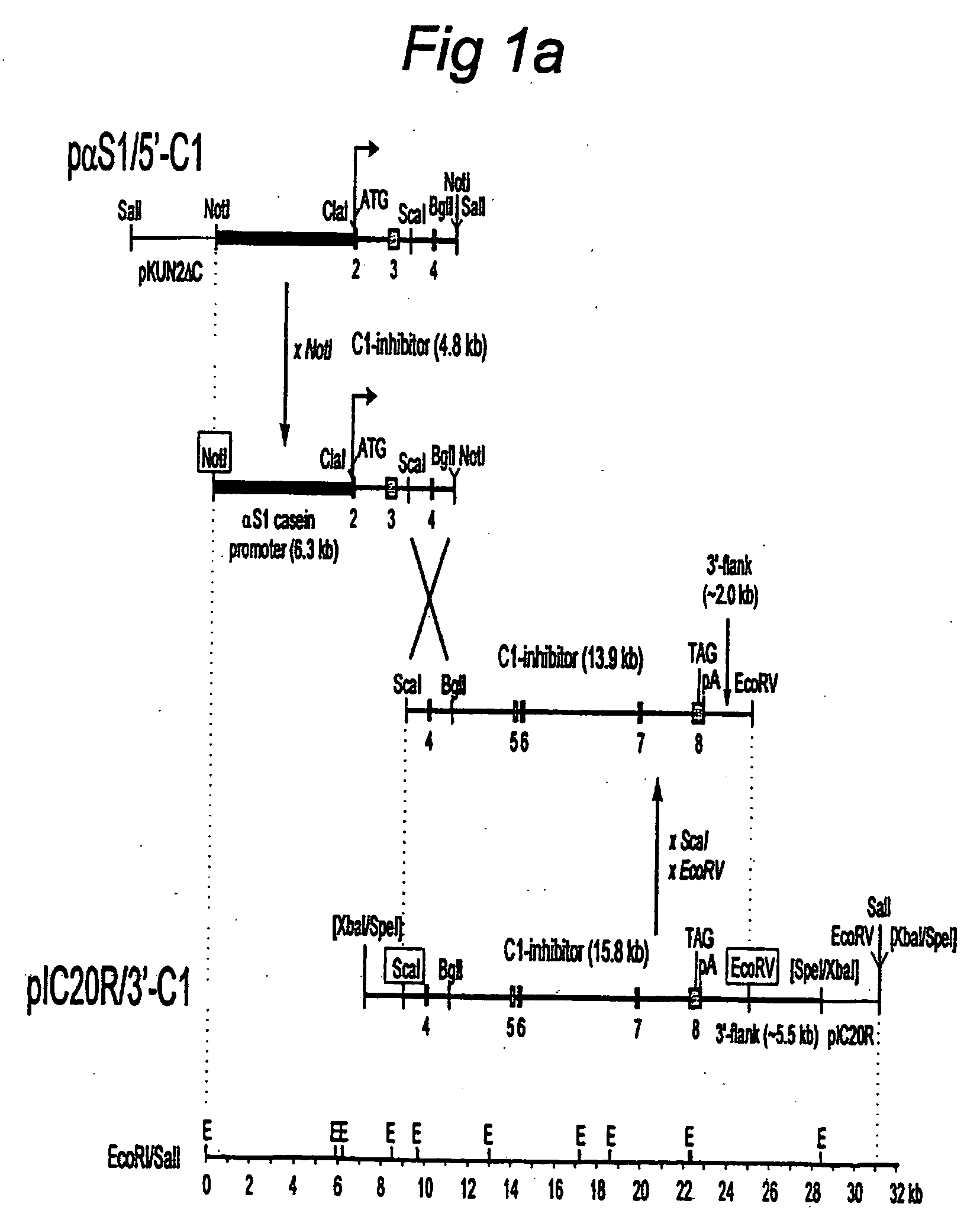
[0065] A set of two expression vectors containing overlapping parts of the genomic sequence of the human C1 inhibitor gene was constructed. Together these plasmids contain the bovine αS1-casein promoter and the complete human C1 inhibitor genomic sequence. All C1 inhibitor fragments used were derived from P1 clone DMDC-HFF#1-1112-69, obtained from Genome Systems Inc. (8620 Pennell Drive, St. Louis, Mo. 63114), which was isolated from a P1 human genomic library by PCR with two C1 inhibitor specific primers (Appendix 1A).
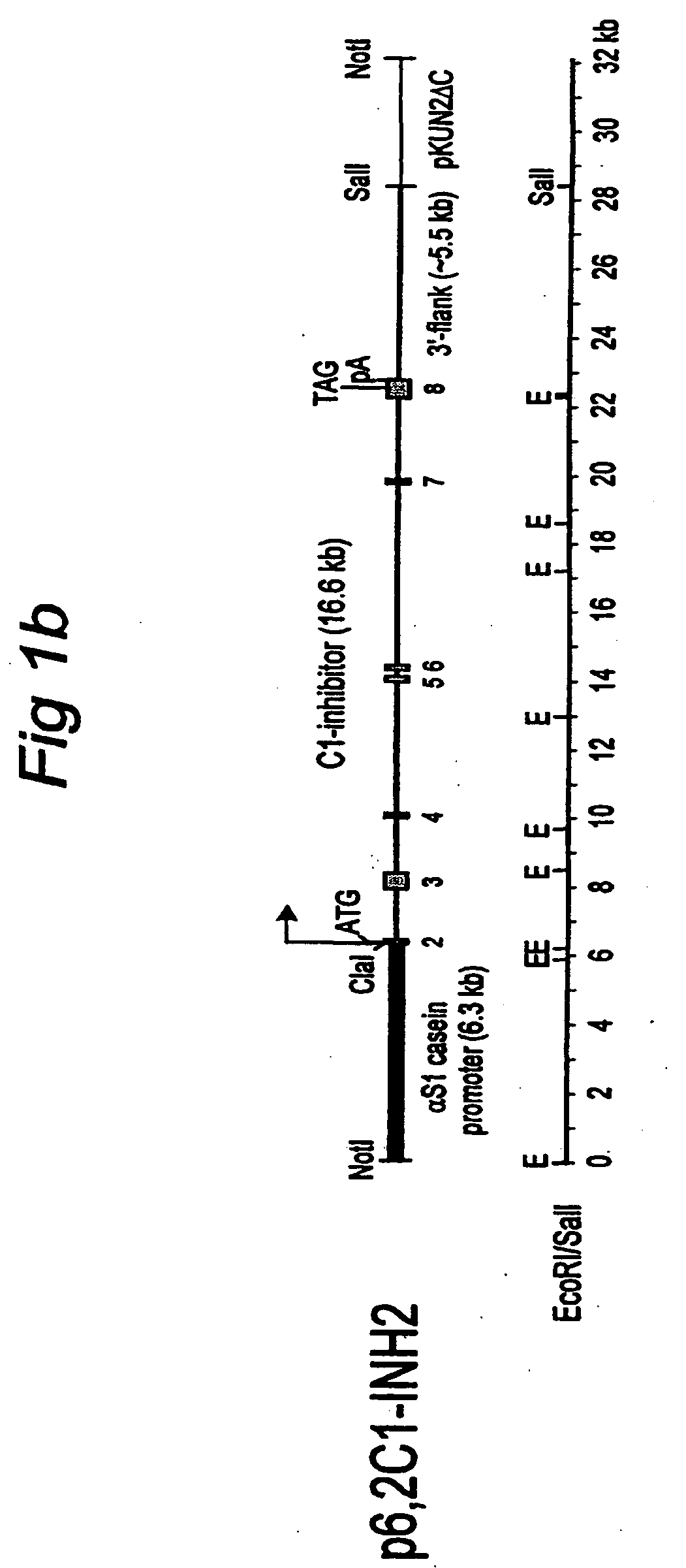
[0066] Plasmid pαS1 / 5′C1, which includes 6.3 kb of bovine αS1-casein regulatory sequences fused to the 5′-part of the C1 inhibitor gene, was constructed as follows. First, pKUN1 [Konings, 1986 Gene 46,269-76] was digested with EcoRI and SalI and ligated to linker 1 (Appendix 1B), followed by removal of the ClaI site by filling in with Klenow and ligating. From the resulting plasmid pKUN2ΔC, pKUN2ΔC...
example 2
A. Overlapping Constructs (CINH1) in Mice.
[0069] Transgenic mice were produced by pronuclear injection of fertilized oocytes, essentially as described by Hogan et al., 1986, “Manipulating the Mouse Embryo”, Cold Spring Harbour Press NY. pαS1-5′C1 (see FIG. 1A) was digested with NotI, yielding an 11.3 kb fragment which was isolated by gelpurification and electroelution. Similarly, a 15.8 kb ScaI-EcoRV fragment from pIC20R / 3′-C1 , extending from intron 3 to 2 kb beyond the last exon, was prepared. Both fragments were combined (at a concentration of 3 ng / μl) and injected into the pronucleus of fertilized mouse oocytes, which were implanted in the uterus of pseudopregnant mouse foster mothers. The offspring was analyzed for the insertion of the human C1 inhibitor genomic gene construct by Southern blotting of DNA isolated from clipped tails. Whether correct homologous recombination between the overlapping fragments had occurred, was checked on Southern blots and by PCR. 3...
example 3
Analysis Of C1 Inhibitor in the Milk of Transgenic Animals
A, B. Overlapping and Single Genomic Constructs in Mice (CINH1 and CINH2).
[0074] Milk from transgenic mice and non-transgenic controls was analyzed by an enzyme-linked-radio-immuno-assay (for a description of the ELISA, see Appendix 2; for the expression data see Tables 1, 2 and 3). The ELISA measures the total amounts of C1 inhibitor (both active and inactive). The average levels of C1 inhibitor obtained in the transgenic mice made with the overlapping fragments ranged from 0.04-5 mg / ml. Some individual samples of the highest producing lines contained more than 20 mg / ml. The average levels of C1 inhibitor obtained in the transgenic mice made with the single fragment ranged from 0.1 μg / ml-10 mg / ml. Some individual samples of the highest producing line (5903) contained more than 20 mg / ml.
TABLE 1C1 inhibitor expression data of rH-C1INH1 mouse linesC1 inhibitor# integrationexpression F1 μg / ml1Line nositessublineAverage2Max3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com