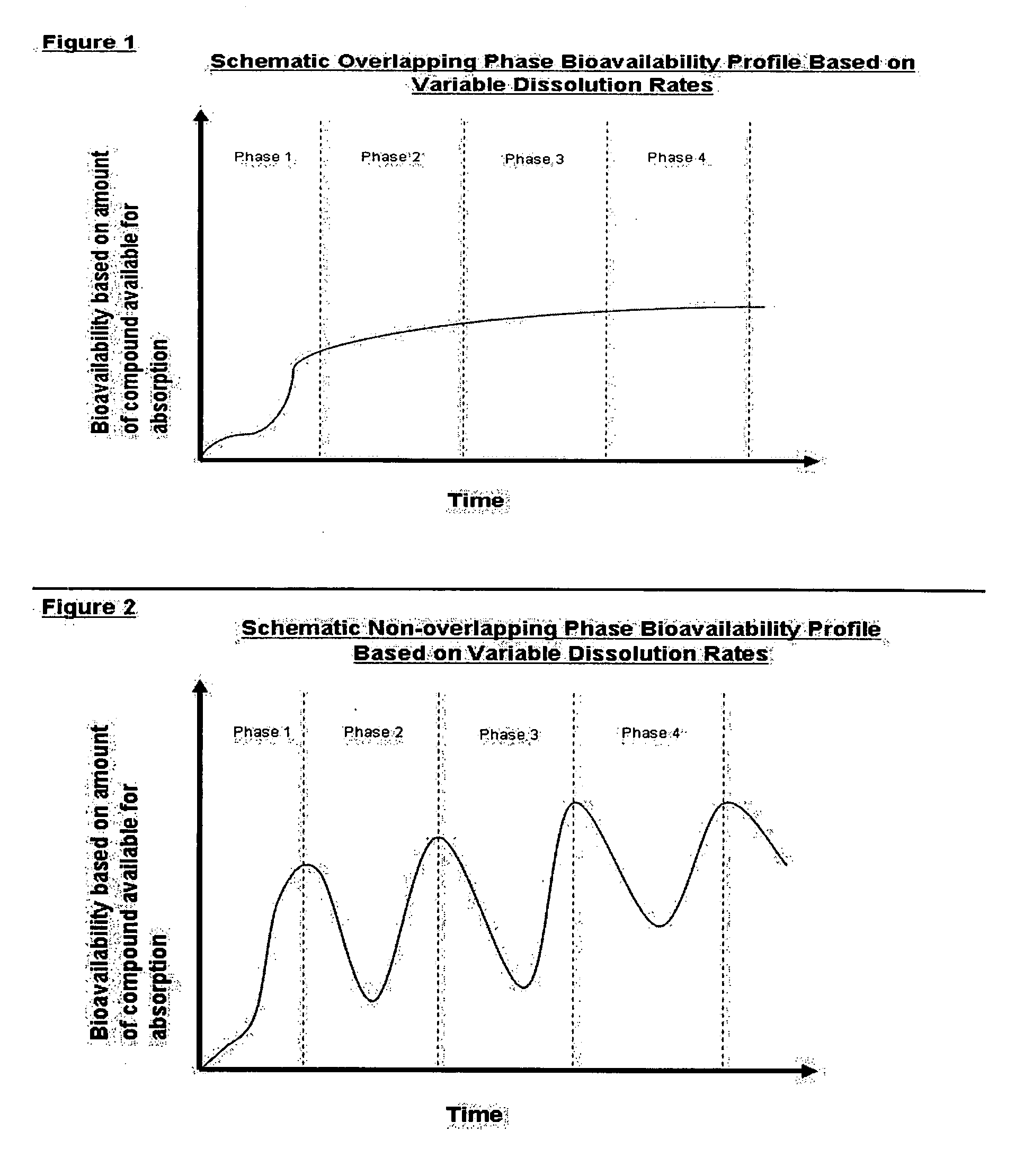
Method for a supplemental dietary composition having a multi-phase dissolution profile
a technology of dietary composition and dissolution profile, which is applied in the direction of drug composition, dispersed delivery, metabolic disorder, etc., can solve the problems of not only addressing the problem of but also presenting a problem in terms of bioavailability, and nothing in the u.s. pat, so as to increase the rate of dissolution of poorly-soluble compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In Powdered Form
[0082] A dietary supplement comprising the following ingredients per serving is prepared for consumption one time per day per individual:
[0083] about 2.0 ng of fine-milled Glycine, about 2.0 g of regular Glycine, about 5.0 ng of fine-milled L-Arginine, about 5.0 g of regular L-Arginine, about 3.0 ng of fine-milled Calcium-KIC, about 3.0 g of regular Calcium-KIC, about 2.0 g of Maltodextrin, about 1.5 g of Citric Acid, about 80 g of Dextrose, about 0.5 g of Sodium Citrate, about 0.3 g of Sodium Gluconate, about 0.4 g of Polyvinylyrolidone, about 0.1 g of Modified Food Starch, about 0.4 g of Syurp Solids, about 0.03 g of Gum Acacia , about 0.05 g of Silicon Dioxide, about 0.027 g of Acesulfame-Potassium, and about 0.01 g FD&C Red #40.
[0084] Preferably, the nutritional composition is consumed in accordance with the following directions:
[0085] Directions: As a dietary supplement, take one serving (35 g) of product before a high-intensity workout. Mix in a shaker cup...
example 2
[0090] A dietary supplement comprising the following ingredients per serving is prepared for consumption one to four times per day per individual.
[0091] about 7.5 g Leucine, about 0.0004 g fine-milled Leucine, about 0.05 g Calcium-KIC, about 0.45 g Hydroxyprpoyl Cellulose, about 1.75 g Microcrystalline Cellulose, about 0.18 g Croscarmellose Sodium, about 0.03 g Calcium Carbonate, about 0.12 g Vegetable Stearine, about 0.06 g Magnesium Stearate, about 0.06 g Silica, about 0.03 g Magnesium Silicate, about 0.306 g Coating [Polyvinyl Alcohol, Polyethylene Glycol, Talc, Titanium Dioxide, Riboflavin, Soy Lecithin, Polysorbate 80, Hydroxypropyl methylcellulose, Colorings], about 0.001 g Lysine Ketoisocaproic Acid and about 0.0004 g Sweeteners.
[0092] Preferably, the nutritional composition is consumed in accordance with the following directions:
[0093] Directions: As a dietary supplement, take 1 serving (6 caplets) first thing in the morning. On workout days, take 1 serving immediately be...
example 3
[0094] A dietary supplement comprising the following ingredients per serving is prepared for consumption one to four times per day per individual.
[0095] about 2.0 g regular Creatine-Ethyl Ester HCI, about 0.001 g fine-milled Creatine-Ethyl Ester HCI, about 0.1 g Creatine Alpha-ketoglutarate and about 0.1 g Alpha-lipoic Acid.
[0096] Preferably, the nutritional composition is consumed in accordance with the following directions:
[0097] Directions: As a dietary supplement, take two servings per day, e.g., one serving (2 caplets) in the morning and one serving (2 caplets) in the afternoon. Consume 10 8 oz. Glasses of water daily. To maximize results, use in conjunction with weight training.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle sizes | aaaaa | aaaaa |
| spherical volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com