Polynucleotides for Reducing Respiratory Syncytial Virus Gene Expression
a technology of respiratory syncytial virus and polynucleotide, which is applied in the field of polynucleotides for reducing respiratory syncytial virus gene expression, can solve the problems of no specific antiviral treatment licensed for public health use to prevent diseases associated with rsv infection, and no specific antiviral treatment available. , to achieve the effect of reducing the expression of one or more rsv genes, reducing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
siNS1 Inhibition of rgRSV Infection
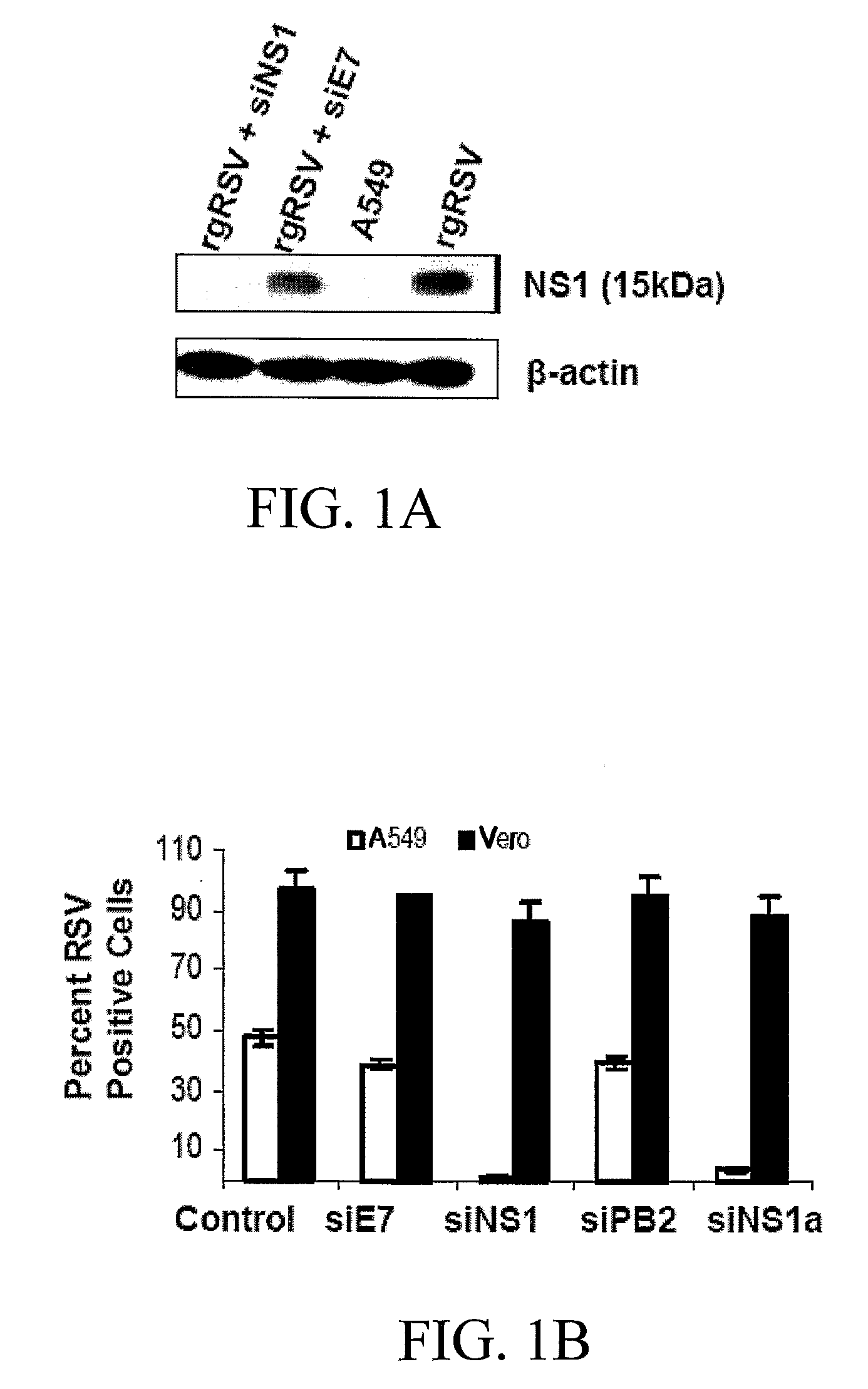
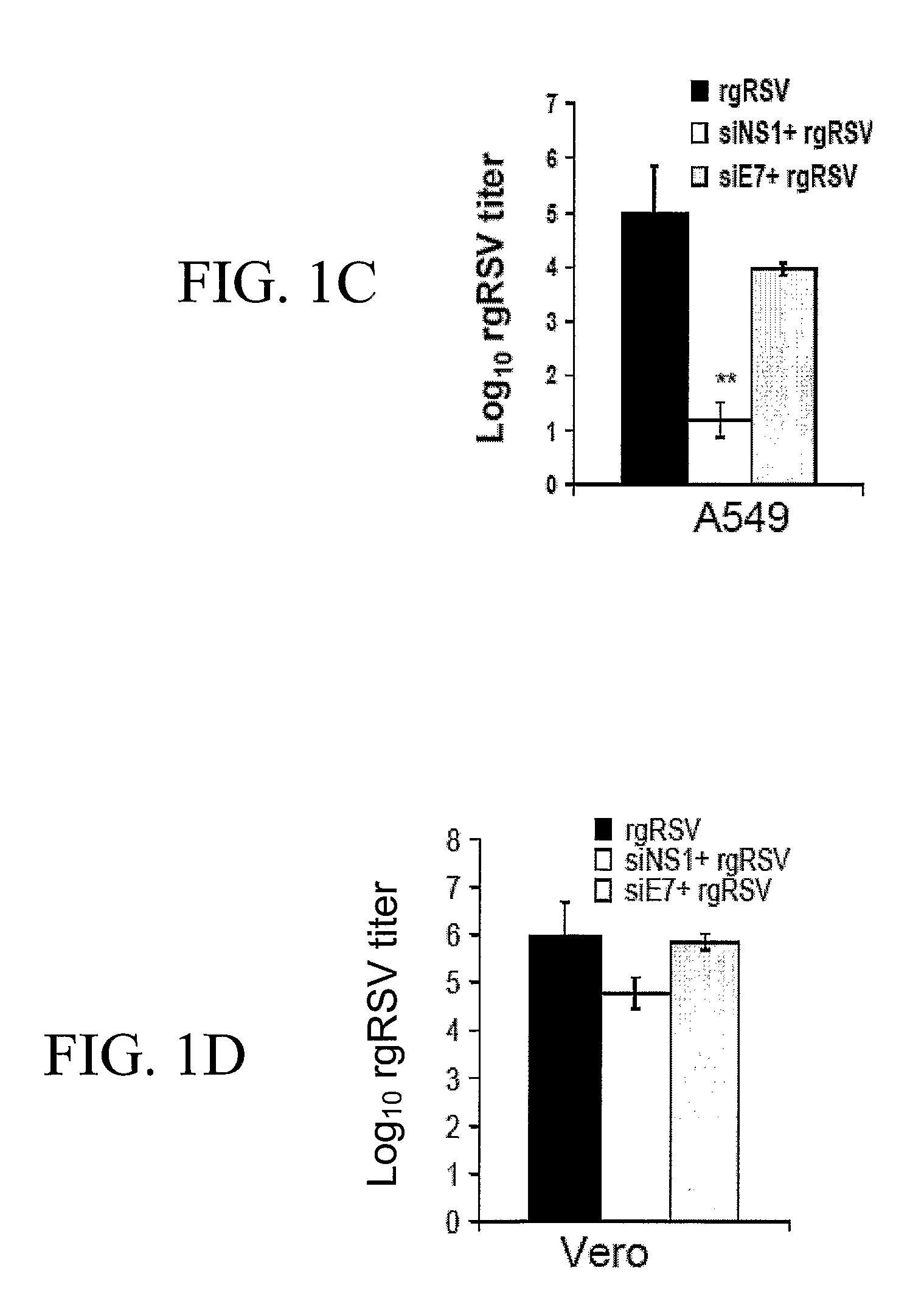
[0146] Two different siRNA oligos for RSV NS1, siNS1 and siNS1a, HPV18E7 (siE7) and Influenza virus PB2 (siPB2) were designed and cloned into the pSMWZ-1 vector (Zhang, W. et al. Genetic Vaccines Ther., 2004, 2:8-12). Analysis of EGFP expression in A549 cells co-transfected with pEGFP and siNS1 / 1a, siE7 or siPB2 demonstrates that none of siRNAs silence the EGFP gene (data not shown). Immunoblotting results show that pre-transfection of A549 cells with siNS1, but not siE7, significantly reduces the expression of NS1 proteins (FIG. 1A), but not that of other viral proteins (data not shown). To test whether siNS1 attenuates virus infection, A549 cells and type-1 IFN deficient (Mosca, J. D. and Pitha, P. M. Mol. Cell. Biol., 1986, 6:2279-2283) Vero cells were transfected with the siNS1, siNS1a, or control siRNAs, and then infected with rgRSV (Hallak, L. K. et al. Virology, 2000, 271:264-275). The results of flow cytometry show a significant decrease i...
example 2
Mechanism of siNS1-Mediated Upregulation of Type-1 IFN Pathway
[0147] The finding that RSV infection of A549 cells, but not Vero cells, is affected by siNS treatment suggests a role of NS1 protein in the promotion of RSV infection by inhibiting the type-1 IFN pathway.
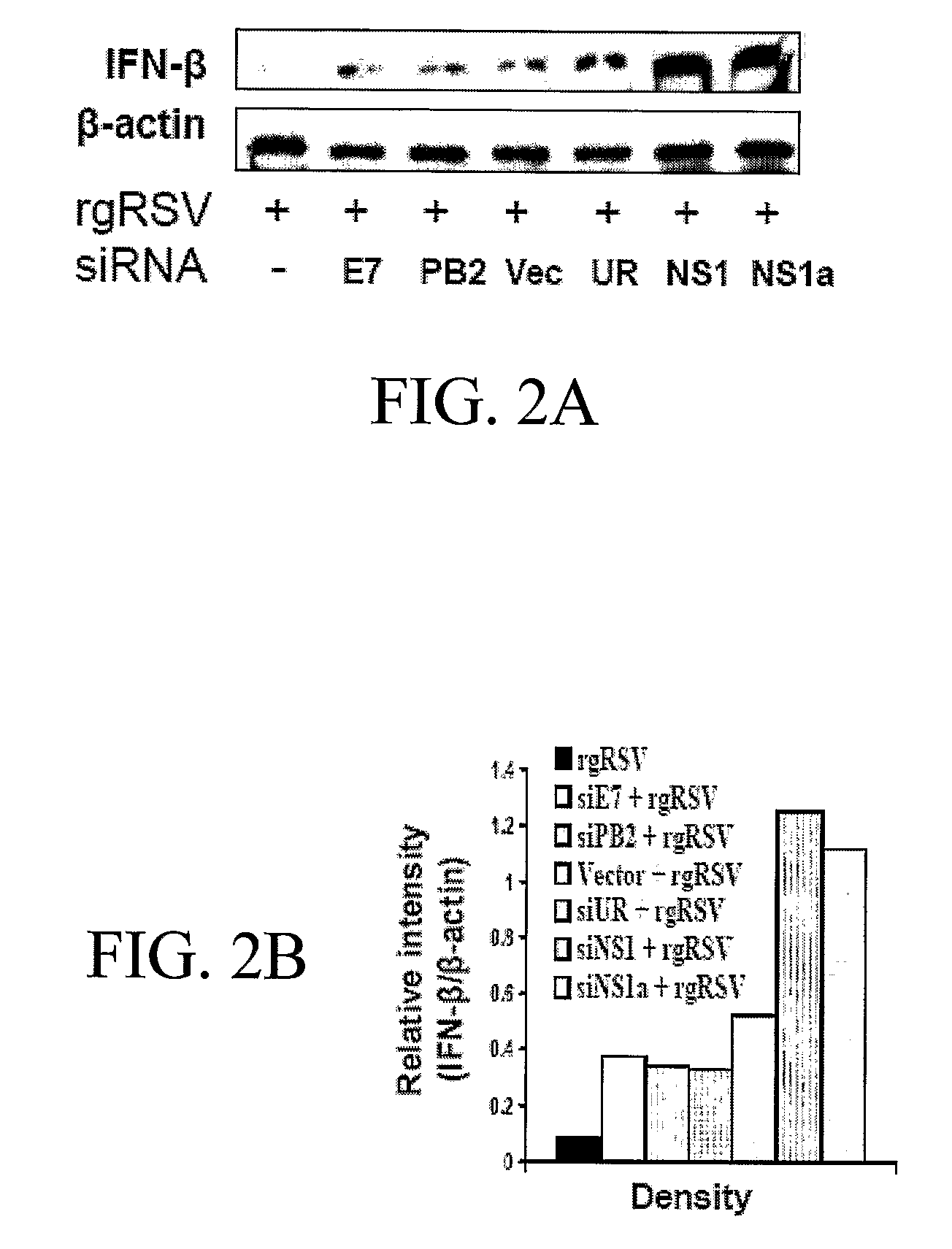
[0148] To verify whether NS1 decreases the amount of type-1 IFN, the expression of IFN-β was examined by immunoblotting. The results show that A549 cells transfected with siNS1 or siNS1a, upon RSV infection, produce significantly increased amounts of IFN-β, compared to the different controls, including totally unrelated siRNA with no homology to mammalian genes (siUR), (FIGS. 2A and 2B).
[0149] To further examine the role of NS1 in regulating the IFN pathway, RNAs from control and siNS1-transduced cells were isolated and subjected to microarray analyses. The results show that siNS1 treatment increased the expression (≧6 fold-change) of 25 IFN-inducible genes compared to rgRSV infection alone (Table 1), and the expressi...
example 3
Silencing NS1 Polarizes Human Dendritic Cells Toward a Th1-Promoting Phenotype
[0151] Monocytes isolated from human peripheral blood were cultured with requisite cytokines to test whether siNS1 expression affects RSV-infected DC activity. Thus, the IFN-α and IFN-β concentrations were measured in the supernatants from cultured, infected, monocyte-derived DCs transfected with siNS1 or control. The data show that siNS1 treatment induces a significantly higher production of both type-1 IFNs in infected DCs than it does in controls (FIG. 3A). Furthermore, to assess the effect of siNS1-treated DCs on T-cell function, allogenic naïve CD4+T cells were co-cultured with RSV-infected DCs treated with or without siNS1. The results of intracellular cytokine staining showed an increase in IFN-γ and a decrease in IL-4 secretion in naïve CD4+T cells for siNS1-treated, RSV-infected DCs, compared with controls (FIG. 3B).
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydrophobic | aaaaa | aaaaa |
| biocompatible | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com